Application of 3D Bioprinting & Biomaterial Technology for Translational Regenerative Medicine
[MUSIC] This program contains graphic images and discussion of medical procedures. Viewer discretion is advised. [MUSIC] I'm very happy to have Jin round and when we were discussing about what kind of seminars that we should have, we thought that it's time for a heavy bioengineer to come and talk about the interface or the interplay between stem cells and bioengineer.
I had Jin in my lab and said he's the perfect guy for that and he's being showing us what he can do in Korea in the lab and now I said, "Oh my gosh, I need to share this with the world." What you're about to learn is really fascinating. I'm sure you're going to enjoy a lot.
Jin is a co-founder and CTO of T&R Biofab. It's a company in South Korea. He's also associated professor of the Department of Mechanical Engineer in Tech University of Korea. Jin is not your traditional stem cell biologist, he actually has a BS in Mechanical Engineering from the Pusan National University, again in South Korea, and he did his PhD Postdoc also in South Korea.
That PhD was also in mechanical engineering with a thesis regarding the rapid prototype for two-dimensional cell printing system and its application to regeneration of heterogeneous tissue. That thesis is basically what he took to do it on his company and he's moving on to more and more complex tissues, more and more situations where he can have a high impact on regenerative medicine. You're going to hear some examples of how he can translate these fast-paced technology to hospitals in South Korea and more and more around the world. The numbers are fascinating because I'm used to hear cases of one or two patients.
He's talking about thousands of patients that already receive this technology. It's a very clinically oriented and we have lots of clinical data to support not only feasibility, but efficacy of his tools and technology. You might ask why he's here doing a sabbatical in my lab.
So that's the funny story. I went off on my first postdocs, who is a bioengineer. I started my lab with a bioengineer guy, Apollo Marina. He finished the postdoc here with me and then he move on, on the industry side that was always what he wanted to do and he ended up working for Jin in South Korea. At one point, we were just discussing and asking, can you update me what you're doing? Because he's always on the engineering side, bioprinting, things like that. He said, " I'm working with this guy.
It's fascinating the kind of technology that we have and I haven't seen that anywhere else even in US." You need to know him. He was able to bring Jin here to US and we visit, we connected right away and we said, I think his original plan was to go to UCLA. I convinced him to come to San Diego to stay with us.
Jin is learning more about the brain and interface of microfluidic chambers that we use to grow very organized things like that. Here's involved already in several projects in the lab and we're having so much fun here. With no further ado, please welcome Jinhyng Shim. Thank you. [APPLAUSE] Thank you very much. Very kind introduction.
My name is Jinhyng Shim and I would like to talk about the 3D printing technology and biomaterial for the tissue engineering and regenerative medicine. Thank you very much for having me here. I hope you enjoy my presentation. Part 1 of my presentation is an introduction to myself and my research background and company and university that I worked for.
I was born and raised in Korea. My major, as Harrison explained, is mechanical engineering for my undergraduate to the PhD. Right after getting my PhD degree, I founded a startup company named T&R Biofab, which commercializes 3D printing technology for the regenerative medicine. I'm also associate professor at Tech University of Korea.
I recently joined the Muotri Lab as a visiting scholar because I'm a subbatical. Let me briefly introduce the university that I worked for. The Tech University of Korea was founded by the Ministry of Industry of Korean government in 1998. The number of students is 7,500, which is a small and mid-size and engineering oriented university compared to other university in Korea. Our school is located in the middle of the industry complex, where there are almost 13,000 manufacturing oriented companies get together.
Great thing about our university is that there are many opportunity for the industry oriented project. Students can also get many opportunity to learn the practical skills while internship at nearby companies. As you can see in the bottom, this is the University located in the middle of the industry park. It is the largest industry park in Korea.
For the sake of the audience who are not familiar to the Korea, we are here at San Diego. Korea is 17 hours ahead of here in California. Korea starts the day earlier than any other country in the world. The time in Korea is now almost 3:00 a.m of tomorrow 9th of the December. I just compared Korea to the California.
California has a four times larger land area than Korea. The population of California is almost at 40 million and Korea has 50 million. Especially nearly half of the 50 million of Korean are living in the Seoul metropolitan area. Therefore you can easily guess how Korea is crowded. The people in Korea are always busy with heavy traffic around the city and they study and work hard because the cooperative environment and competative to survive or feared of their life. Some people think that is a driving force of the Korean growth.
However, I feel sometimes too much. That's why I'm here at San Diego. [LAUGHTER] How I can love the San Diego here. Less competative, less crowded. So I love San Diego. Let's go back to the work.
I try to talk about the tissue engineering today. Everybody knows here may know the tissue engineering is. The tissue engineering is an interdisciplinary field that applies the principle of the engineering and life science. The ultimate goal of the tissue engineering is tissue or organ regeneration. Especially I try to focus on how to make a three-dimensional scaffold using 3D printing.
Mainstream component of the tissue engineering is your cell, scaffold and growth factors. As a mechanical engineer, I have a perspective and a viewpoint of the looking at the tissue engineering as similar to the building a skyscraper in architectures. I think the main actor of the organ and skyscrapers are cells and human respectively. Things that make the main actor forms three-dimensional shape are the extracellular matrix and these materials like steel beam, steel bar, concrete. The growth factors and community futures play a significant role in increasing the live coverage of the main actors and organs can live or function properly without a blood vessel and nerve.
Similarly, human can live long without water, air circulation and electricity and telecommunication. That's why it is very challenge to regenerate functional and complicated tissue, like a heart, liver, and kidney. If we see the 3D printing technology, the 3D printing is one of the techniques to manufacture the 3D object using a layer-by-layer process. It is called additive manufacturing. If we see how the 3D printing contributes to tissue engineering is, some researchers print out cell Type A, next to the cell Type B.
Then they found that the two different types of cells were fused and assembled, even they formed a three-dimensional tissue-like structure. Then people start to think that if we could position the cells in the desired three-dimensional space, we could get a three-dimensional structure consisting of living cells. If we find a way to create your cell structure, we could get a tissue or organ. That is the idea of the 3D printing for the tissue engineering and regenerative medicine. The role of the 3D printing for the organ regeneration could be very similar to that of the crane on the construction site.
Crane moves, and upraise the thing that is needed in building a structure. As a mechanical engineer, my research journey for the tissue engineering past 15 years starts from the development of this 3D bioprinting that I needed. This 3D printer has been developed by ourselves from design to assembly, software to hardware. It has been commercialized since 2014. Since then, we have updated every year up to 2018. We can make a three-dimensional printer on demand, depending on the application we want.
This slide shows how the 3D printer looks like. It has x, y, and z axes low boric stage system and has multiple printing heads. These multiple printing heads allow us to print different types of biomaterials like a polymer, which is a thermoplastic and hydrogel a very low viscous culture media, which too can encapsulate living cells.
We can have the different types of the working plate depending on the application. The process is fully automated, and we adapt the parts which can keep the environment aseptic. This is the bioprinter we designed for the use of the cell creature available GMP facility, which was already installed at the Seoul St. Mary's Hospital in Korea for the purpose of the research to print out the trachea tissue for the clinical application. Using this kind of printer, it can be confirmed that the three-dimensional very complicate the structure can be manufactured as we planned in advance using various biomaterial and different cell types into three dimensional. This is the one example how the 3D printed scaffold looks like.
As you can see here, there are many pores, and the pores are fully interconnected because it is produced by the layer-by-layer process. This scaffold consists of the biodegradable polymer like a polycaprolactone. We tested In-vivo test using various cell types from mouse to human.
The cell can adhere on the structure and can proliferate around the structure. We also perform the In-vivo test for bone regeneration. The scaffold was designed, as you can see on the left-hand side, the porous scaffold was first printed out using a PCL, polycaprolactone and tricalcium phosphate material. Every other pore was filled with extracellular matrix that can encapsulate the bone morphogenetic protein-2 grasp vectors. Image at the top of the right-hand side shows the 3D-printed scaffold that contain growth factors.
Graph at the bottom shows that the encapsulated BMP-2 was slowly released over the 28 days. As a result, In-vivo, as you can see in CT and H&E staining, active bone regeneration was confirmed in the groove of the scaffold consisting of extracellular matrix and gross factor in covariate effect of rats even in four weeks. It was so dramatic, and it actually led us to make up our mind to commercialize the technology. Based on this scientific background, my PI Professor Donald Cho, and current CEO Dr. Yun and I decided to found a company named T&R Biofab to commercialize the technology we developed from the bench to the bedside. T&R Biofab stands for the tissue engineering and regenerative medicine bio-fabrication.
From the beginning, our business and target was to commercialize the polymer scaffold, extracellular matrix product, and tissue organ printing. Let me briefly introduce the key numbers on T&R Biofab. We established the company in 2013, and 2014 we first applied our product to the patients, 2018, the company has been listed in the Korean stock market. Now, the company is a public company, 2022 is the tenth year of the company history.
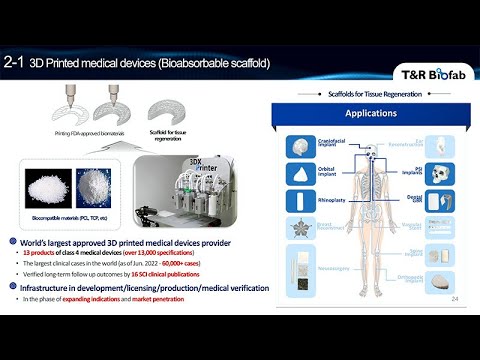
We have 171 intellectual property rights, and we secured a $50 million grant from the government. We have raised to fund $100 million so far. We have 100 employees, and as of now, the market cap of the company in the stock market is at $300 million. Let me show you the product we have already commercialized on the market. First one is a 3D-printed medical device. This product is called a scaffold.
We successfully commercialized the scaffold first, and this is categorized into the medical device. However, it is not the same as that I showed you previously. To simplify the regulatory process, we just used a non-biological material like polymer, and inorganic ceramic. We just excluded the extracellular matrix and protein first.
These scaffolds are already on the market. If you see the numbers, we have 13 products in the market. We have almost 60,000 patients who already are wearing our scaffold, and we published 16 SCI clinical publications. This is the scaffold we have, we have different shapes of scaffold products from the patient specific to just a general mesh which can be manufactured using just mass production methods, just mass-producible. Let me show you the representative case for the patient-specific implant. This patient was suffering from the scleroderma disease, it was that there were cibia asymmetric depression on the left side of the face and skull of the patient.
The patient wanted to reconstruct this asymmetric depression. We analyzed the patient's current condition using a CAD modeling technology and designed three different patient-specific implants. This is the scaffold we print out. The scaffold made up the polycaprolactone and tricasion prospect, which is just ceramic powder which will help to regenerate the bone. The scaffold was designed to have a large size pore to allow the surgeon to pack the various bone material into the scaffold to increase the possibility of the bone regeneration. Plastic surgeon collaborating with us have begun to understand the technology is better.
Surgical technique has been improved a lot. Recently there are various clinically or variable biomaterial have been used with the scalp fruit. Indeed, in this case, patients autologous bone, artificial bones substitute and BMP-2 cross vector and allergenic bone human dermal matrix and all relevant biomaterial have been mixed with the mix and apply with the scaphoid. Decision, put the material into the scalp fruit and scaffold payers just of payroll as a framework to put together in our body.
It was very challenging case, but the research was very successful. New bone formation was absorbed in one month after the surgery. If you see the another CT image, we can see something added at the defects side here, here, here in a one month post operative CT image. The CT image likely absorbed and detect the implant with the artificial bone and otter lovers patient's own bone chip.
We were very happy with the result after one month, but we had to wait and see how these things goes well. Clinical outcome after three years was better than we expected. The depressed the facial contour has been augmented. Interestingly, we observed a parent bone regeneration at the cranial part and newborn looks to fuse the well with the native bone at the cross-sectional view of the CT image. It was very successful but we have almost as 300 just patient-specific implant cases in Korea and I bring one more interesting case to share with you.
Actually, it has been done last month. Colleague at Wake Forest Institute for the region, Madison ask us to print out the patient-specific implant for the American soldier who owned it from the Ukraine war. They sent us some patients image and CT data, it was like that. As you can see here, there was a very significant defect at the right side of the skull.
I have no idea what happened to him but somehow patient had a severe skull defect and despite the severe injury, he fortunately survive. The recast that I received was to manufacture the patient-specific implant as soon as possible, followed by the Korea Regulatory Process and send them to the Ukraine. But we couldn't find a way to send the things to the Korean directory, so we send it to the Poland first and someone carried the Material to the Ukraine. We designed later based on the patient's CT data and this is the image. We confirm that the 3D printed the implant is a well fitted to the skull defect and in front with a stirrer wise and delivered to the Poland first, and it has been finally delivered to the Ukraine but I haven't received a result yet.
If I have another chance, I will share with you guys. As you can see the patient specific cases that are always very dramatic and exciting. However, is true that it is very difficult to secure Steve cash flow from this the patient's specific. We tried to find a way to make it stable in terms of the business so this project came to us from the B Braun.
When the craniotomy is required to the various diseases such as a brain tumor, brain stroke, or just brain infarction post-operative bone defect cannot be avoided like this. This bone defect causes a softer tissue deformation, resulting in an unsteady factory appearance. Recently, patients who values and care the quality of life after the surgery are sensitive to this outcome.
Doctors should consider ness important brain treatment and aesthetic outcomes after the surgery. The B Braun, our distributing partner, came to us and ask us to collaborate to overcome these unmet needs after identifying the demands of the field. It was very successful when we project for both companies. We use the 3D printing technology and approved the material to make this skull fold what they wanted. Over year T&R Biofab completed every process from design to getting approval from the government, FDA and the B Braun generated additional cells through the essays and metric.
This case was an excellent example of the confirming that the possibility of creating a stable cash by applying 3D printing technology, not only for the patient-specific but for the mass produce material product. Let's move to the second product we developed and recently we launched the material on the market, which is extracellular matrix based bio surgery product. Tissue in our body are composed of the cells and extracellular matrix. In other words, the extracellular matrix is a critical component other than a cells and is responsible for the physical structure of the tissues. In 2014, we first apply the decellularized extracellular matrix material to the bio ink of the bio printer and published in Nature Communications.
We found that the tissue specific decellularized extracellular matrix was the idea for the bio ink material. Despite the outstanding scientific outcomes, there are bio ink itself was not enough to make some stable business opportunity. One day, one idea came out, why don't you apply our DCM material to wound healing ointment, which is relatively simple and it's market size is extensive and easy to approach the customers. It was actually a good idea, it worked.
We applied the decellularized extracellular matrix, especially derived from the person aorta, the blood vessel. This free dECM consists of 40 percent of collagen and 60 percent of the elastin. This material helped to have a dual function of the enhancing wound healing and scar tissue reduction even though the amount of the ingredient is very little, one or two per cent of the ointment. Last other 98 percent of the material is already there was no material.
It was launched in Korea, Micah from last July and we also tested some test the blood vessel DCM help to induce angiogenesis, resulting in faster re API sterilization. It has a rapid wound healing and reduce the scar regeneration. Actually there's one story behind this. When you go to the government office to get the approval for the regulatory up there at that time, we did not emphasize that our product is a very much innovative because to claim it is just identical to the product on the market. If we claim it is very innovative, very rocket science, that time we have to prove that efficacy that node t, using tons of the documentation. To avoid that situation, we just claim this is a very normal and it's not special.
It was our strategy when you get an approval. But I think it worked because we could get the approval less than one year. We are currently establishing our commercialization strategy in the direction of improving performance by applying our proprietary material to the product already in the market, like an ointment type of the wound healing product, and I said it's a dermal matrix and until additional barrier something. This is the strategy we have. As you can see from the table, we have a plan to launch the new product every year until 2025.
So far, everything is on the right track. Let me introduce our third product, cell technology, cell therapy. Everybody knows what the iPS is, so from our tissue, from the patients, if you treat with a special factor, we can make pluripotent stem cell, and that pluripotent stem cell has a potential to make or re-creation of any cell or body. Through the collaboration with the plastic surgery university hospital, the patient tissue was obtained and skewered and iPS cell line was established from the tissue. At that time, all document work, including patient constants was conducted according to the US FDA standards and differentiation of the cardiomyocyte, hepatocyte, and vascular endothelial cell was successfully confirmed using our T&R iPS cell line.
This slide shows the cardiomyocyte differentiate from the our T&R biopower iPS. At that time, the iPS was prepared using mRNA based kit without a user of the viruses, rippling cells on the surface of the cranium media indicate that differentiate the cardiomyocyte hybrid nature of the bidding, and it was a purified by our specific marker for the cardiomyocyte. After that the patch containing a cell with a print out using our printer, and then we directly attached the patch to the site of the myocardial infarction.
However, open heart surgery is unnecessary and avoidable to apply this method, and we thought that it would be the big harder for the commercialization. We had to find another way more easily from the regulatory point of view, a method of injecting only cells without a patch into the myocardial infarction site using anecdote was tested. As you can see this product catheter already developed for the drug injection into the heart have been commercialized. In this way, we made an aggregated cardiomyocyte, which in size is about 100 to make it easy to use in a catheter system. In animal test effect of the improving cardiac function was higher in the aggregated cardiomyocyte application group than in the single-cell application groups.
In particular, immunostaining resulted to build that aggregated cardiomyocyte contribute to the regeneration of the cardiac tissue while stably remaining in the body for a long time. This is our heart, mini organ and bid, which is a bidding, I love to see this movie all the time, and nowadays we are trying to move to the large animal tasks using that cardiomyocyte. I will share with you the next chance, and using our bioprinting technology, we can create a three-dimensional cell structure, which has a very complicated and a small size of structure like liver.
As you can see in the picture, this printed structure indicate a liver structure and this green part and red part of the structure means the endothelial cell and the size of the sphere and the structure is less than one millimeter. As we planned and we engineered, we can praise the different cell types as we desired, as we wanted. As you can see, this green part in blue part indicates the hepatic cells and T's network type the cell looks indicate the endothelial cells. Even in the case of the simple spear mentioned above. Different cells can be printed into the desired pattern.
Even inside of the speaker aggregation. Looking at the research published in the cover paper by localizing the vascular cell outside and inside into the 3D shapes. This 3D printed spear was transplanted into the body of the animal. At that time, we confirmed that the cell did not die early due to the lack of the blood vessels and even the cell fused with the surrounding tissue while remaining in the body for a long time. It is believed that this would be the great advantage to the technology that applied to the 3D cell organoid as a cell therapy. Let me show you the conclusion of the presentation.
Our Channel Biofab has its technology on: 3D printing scaffold, biomaterial, and stem cell. These technologies can be commercialized individually, but each can be connected to make advanced innovations. In addition, we kept pursuing a way to commercialize the technology in tissue engineering and regional medicine to make a value. Sooner or later, we hope to see our products sold in the US market. We will continue to explore the opportunity to commercialize that therapy technology as well.
All of these results wouldn't have been possible without our researchers' dedication. We especially thank the researcher and we would like to also thank our collaborating partners like B Braun L'Oreal, the Millipore Sigma, and Johnson and Johnson, especially last April, the CEO, walk into our door visited our R&D center in Korea, and he acknowledged our technology and the collaboration done with the Johnson and Johnson innovation team. We were very proud of that, and we also thank the government grant and especially the investors who have trust us. Without all this investment and support from the government, this research cannot come out.
This is the last page. I sincerely congratulate the Korean national soccer team on advancing to the World Cup round of 16. It's been almost 12 years since we had our last.
We hope you enjoy the rest of the World Cup game matches. Thank you very much for your patience and attention. If you have any questions, please let me know.
Thank you. [APPLAUSE] I see one question already, so bring the mic over here. I was just curious as to, really what effort you have to go through to have an uncontaminated deposition of everything? Meaning how do you keep bacteria, viruses, fungus, mycoplasma out of your preparation? Meaning of the process in 3D printing, actually, there is a special treatment when you treat the biological material in 3D printing. For example, in the last page, we equip the 3D printing housing as a say curvature bench type disk, a curvature facility. We set up this air circulation system and it has some air feature system and also, it has a steroid injection system like having us some UV light before we use. They're things can make it the environment clean and keep the aseptic.
Great. We have a question that came on through Zoom and it concerns vasculature. Is it likely that vasculature can be sufficiently printed to satisfy the circulatory requirements of a complete printed organ? It's a good question.
Actually, when we use a just a cell in the animal tasks we usually, put the target cell. But the time though, if we put the target cell into the body, the targets cell cannot survive in our body long because there's a circulation problem and especially circulation problem can be overcome by the some vascularization. That's why we print out the vascularization relevant cells, like endothelial cell to make the target cells survive long.
Even if you use the 3D printer, we can print out the intracellular cell where we want so means that if we need vascularization at the center of the structure, we can print the cell at the center or if you need a cell surrounds the cell that we can put the cell that position. We can control the position of the printing cell as you want. That's the power and that's the beauty of the 3D printer. That's the things we can overcome a current therapy using a surrogate bioprinting. I guess, a follow up to that is even if you put in vascular endothelial cells, which is the perfusion come from.
That printed cellular vascularized structure can fuse with the native vascular. That's the way we connect the artificial one to the native. That's very important.
If you do a transplant, it gets vascularized by the host. But then also if you have artificial or a synthetic printed vasculature, it also hooks up to that. That's very cool. And the chances that the the organoids problem.
Yeah. Questions out in the audience. Thank you. It's just about how did you fit the tissues? How do oxygen and the tissues and then the other question, it is possible for muscle to the bone for example when I can imagine that people who have an accident and they lost bone and they lost muscle for the surgeons maybe could be difficult to take tissue from another part or just to cover the defect? It is possible? Let's see, the first question is oxygen problem of the tissue? Actually, maybe that cache is connect to the previous caches so without oxygen supply to the tissue, that tissue can experience some hypoxic situation. That's why the three dimensional construct always need some vascularization system.
That's why we put the intracellular cell to make it to help to facilitate the vascularization system. That's the things that I can give you the first question and second one is I couldn't catch the details exactly, but if you are meaning of the bone around the connective tissue actually, when we have a defect, the bone does sometimes have connective tissue like muscle and skin can be damaged at the same time. But actually a current stage never overcome the make tissue at the same time, we just focused on the bone first and our surrounding tissue like muscle and skin can be covered by the patient's own tissue which is obtained from the other part of the body. That's the things that we are doing in the field. The next question on Zoom actually, probably as an elaboration of the question we just talked about. Very often when there are injuries of a particular part of the body or even diseases that involves more than just one cell type.
He gave an example of it's not just bone, it's muscle and we talked about vasculature. Certainly, in complex organs like long hard pancreas, brain repairing requires not just one cell type. Yes. Right.
The question is, have you figured out ways to print where there are multiple cell types integrated into that? Yes, we did. We have experience to make a skin and cartridges. Even the skin consists of the multiple layer of the cells, the fibroblast, keratinocyte. We print out a layer by layer process, different cell type like a first we print out the fibroblast and then the keratinocyte on the top. At that time, if we print a different set time at a different position was more effective to make a skin.
We have another example like a chondrocyte are also chondro tissue means it consists of the bone and subtle superficial, the cartridge. That time we print out the different cell types, like a bone cell at the bottom and the chondrocyte at the top. At that time, it wasn't more effective to make an osteon chondro tissue which is heterogeneous tissue.
That's the things that we can do in the 3D printing, the layer by layer process in three dimensional, localize a different cell type. If you have any application, that application, it is a very good example to apply the 3D printing to that 3D construct. Any questions out there? The next question on Zoom follows up on that. The question was interested in asking, do you think that your technology, the printing could actually create a structure as complex as an alveolus, let's say for the lung? That's the most challenging part in long way. It has a very complicated and multiple serotypes like epithelial cells and compress and extra cellular matrix.
That's why it remain very challenge in the long part and so I haven't tried in a long. But I think if we can find a way to collaborate, I hope to experience their part as well. The next question concerns when you implant these structures let's say into a living organism, does that provoke any inflammatory response? Good questions. There is an inflammatory response all the time, actually. Because most of the surgery case have inflammatory response, more or less.
I think we also experienced when we implant the product into the patients, there was an inflammatory response, but there was a no immune rejection. Immune rejection and inflammatory responses are completely different, so the polymer does not cause any immune response. But we can easily see the inflammatory response. But the inflammatory response can be gradually resolved by any treatment of the medicine. It was not that big deal for the application of the product. Other groups using biomaterials and in fact, including Eris, has found that the biomaterial alone will inhibit scarring, let's say in the heart or the brain.
Do you find that as well? That the very act of having that biomaterial there? Yeah. Sometimes the body recognize the biomaterial as a foreign material, so the bodies tends to encapsulate or isolate the foreign material using some encapsulating tissue. At that time we can see the scar surrounding scaffold or it's sometimes so but it is not common.
We have experience to see that situation. The next question follows up on that, and it's do you find that the scaffold alone, even without cells, if implanted, can have a therapeutic effect? Yeah, we did, but as I mentioned in the previous slide, the scaffold itself did not have a very dramatic outcome because the polymer itself just a support as a mechanical supporter, so to invite a cell and native tissue into the scaffold, we need something more. That could be the extracellular matrix or the patient's own cells. That's why surgeon tried to use not only the polymer scaffold, but also a bearable material like a patient on tissue or some commissary or bearable material on the market. Any questions out there? You started off your talk indicating that you had engineered some of the biomaterials to secrete molecules, through trophic factors. Have you continued that approach? Yeah. We nowadays, rather than a bio printer,
we try to find a way to just use the mercury like extracellular matrix protein just a pure protein. I imagine you could also engineer it so that you do have a 3D organ, but you put key molecules on the end that are there forever, that can attract cells, or organize cells is that visible? Is quite new for me actually. Product Number 4.
Yeah, thank you. Another question came in. It concerns the cardiac work. Chuck Mary. He's not the one asking the question, but it's relevant to Chuck Mary's work. I'm sure you know about it up in Seattle, found that when cardiomyocytes are implanted, they created an arrhythmia and he spent a lot of work trying to figure out how to make cardiomyocytes such that they're not arrhythmogenic. Have you found this problem of how do you approach this? That problem happen in the current stem cell approach in the cardio part, so is true.
But one of the group, which is the Benzene Group in Japan, the Heart Seed Group. I forgot the name, the researcher, but maybe Fukuda. They published cardiolipase derive the cardiomyocyte approach to the monkey at that time. At the early stage they found there's some arrhythmia. However, after that, and harmonize the bidding was a synchronized as time goes by. So I don't know what happened, [LAUGHTER] but because the amount of the injecting cell is another major part, so I think that that could be synchronized somehow.
In fact, Chuck gave one of these sessions about a year ago and went into all of those details. I think we've reached the end of our questions coming in on Zoom. I guess my only last question is did you bring your machine with you up analysis lab that we can all use? I wanted, I wished, but is quite big actually. [LAUGHTER] We can make room, we can extend [LAUGHTER]. We actually cant. [LAUGHTER]. Yes. That's why I'm here to find
a good chance to collaborate. I love to see the moment my printer is here. I will figure out with lot of the people here. We'll, work on space for it. We experienced to set up our printer in the Georgia Tech, the Dr. Scott Hollister left, who is expert in a bio printer.
He adapt our printer and we experienced the setup that our printer view just the deliberate, the printer, larger side printer to the Georgia class. Yes, it's absolutely possible. Great. With that,
thank you, Jin, and thanks everybody for attending whether here in the auditorium or around the world Zooming in. Have a safe and happy holiday season and we'll see you in 2023 for what will be the 20th anniversary of the Southern California's Stem Cell Consortium. [APPLAUSE] Bye bye.
Thank you. Thank you. [MUSIC]
2023-01-12 18:01


