The Immune System in Regenerative Medicine
I'm going to talk a little bit about some meandering path in tissue engineering actually starting off in focusing on stem cells and ending up in the immune system. The immune system in regenerative medicine. As you all are familiar with tissue and organ loss remains of global challenge and while organ donation is increasing, and we do have synthetic implants and options for some tissue loss and organs such as hip or knee implants. There's still great desire to have a real tissue substitute. The field of tissue engineering evolved to provide this solution in an essence, using a three-pronged approach.
Where you have a biomaterial scaffold serving as a three-dimensional framework for new tissue growth and also as a framework for stem cells or just regular cells from a biopsy and various factors that might be used to stimulate that tissue growth. What we noticed when I started is that there was a lot of excitement for the field, but there wasn't that much translation. We were very interested in moving something forward to the clinic as early as possible while at the same time studying the basics of stem cells.
One of the key aspects that will come up throughout is what are the key therapeutic factors if you want to translate? If you want to build the best technology, what are the levers that we want to move up and down to actually promote tissue growth and how might they be different in different scenarios, different people? Our focus was on cartilage. Initially, so this is a tissue that lines the surfaces of your joints. Unfortunately when it's damaged, it can't repair very well. I mentioned hip and knee implants and those are widely used.
But ultimately we really do want a biological solution. When I started 20 years ago, it was about the time when stem cells were quite rising in their focus of research. This is picture here of the mesenchymal stem cell was something that was pretty much shown at every conference when I was starting out. This was of course, particularly interest for us looking at cartilage.
We don't want to take a biopsy. Can we take these cells and make cartilage? We build materials to do that. Then there was the embryonic stem cells, and so we spent a lot of time trying to understand how can we take an embryonic stem cell and consistently get it to make cartilage and actually also bone and adipose.
Finally the last stem cell type that we looked at were the induced pluripotent cells. Okhee Jeon did some work with bone making osteoclasts and osteoblasts from iPS cells. But it's been quite a while since we've worked with stem cells in the lab and I want to tell you a little bit more about that.
As we were looking at translating technologies, we wanted to take the information that we learned from our stem cell work. These synthetic hydrogels and which we would encapsulate cells, we can take chondrocytes the cells that make cartilage, adult stem cells, embryonic stem cells, and provide various signals to induce that tissue growth. But at the same time, we wanted to get something in the clinic right away. It was clear that delivering cells, living cells themselves to patients broadly was going to be hard.
We wanted to start off with something that connected with current surgical practice. In order to translate what we learned from the hydrogels in the stem cell work to the clinic, we ended up working with micro fracture, a procedure where you essentially take a pic and drill down into the bone, cause some bleeding and mobilize cells, and the real goal is mobilizing endogenous progenitor cells. This was the basic paradigm. We had to develop an adhesive to help hold the hydrogel in place, so we paint that adhesive in their, perform the micro fracture, and then you have the hydrogel and you can see that essentially that hydrogel is enriching and concentrating the stuff that comes up from that drilling process. Also, it's discouraging fibroblasts or scar tissue growth. It helps in a few different ways.
There were two clinical trials in this. The first trial went for one year, the second one look more at efficacy at two years. If you take microfracture alone and shown in the red, it starts degrading at about 12 months. This has been seen in the literature, as also seen in our patients. But when we have that hydrogel in there to help redirect that healing process, the cartilage is more robust and last longer. One thing we noticed is that in this trial, that cartilage repair trial, we were redirecting the wound healing process.
The biomaterial wasn't serving as the three-dimensional framework for new tissue growth, but it was redirecting that wound healing process. We also had another clinical trial that we were looking at for soft tissue fillers and we did a clinical trial where we made the implants and it was in patients that we're going to have a tummy tuck procedure. But three months before they got that implants, so then we could get the biomaterial back. We noticed some interesting things; depending on which tissue the same biomaterial was adjacent to, whether it be subcutaneous muscle, adipose, or dermis, the immune cells were different. There was a tissue specific immune response to the same material.
This is really interested into looking at the immune system and maybe rethinking the biomaterial response, and maybe what was the first target we could use to promote tissue repair. I went on sabbatical to Switzerland. Thankfully, Jeff Hubbell and Melody Swartz welcomed me to EPFL there, and so I got to at least learn a little bit of a language. Immunology is not easy.
Then when I came back to Hopkins, little did I know that right next to me in the buildings adjacent to me, there was the cancer immunotherapy folks who are really doing groundbreaking work in that area. While they were focusing on dissociating tumors and looking at what immune cells were there, we can apply those same techniques to dissociating tissue spaces where wounds and biomaterials were implanted to understand what was going on in the repair process. The immune system is interesting and tissue repair, sometimes it helps and sometimes it hurts. I have some clips of papers here that show how these cells are required. Macrophages are known to be required for tissue repair. But there are things that associate with negative tissue repair, whether it be CD8 T cells or certain immune signatures in the blood that can predict a fast or a slow recovery.
Now in the case of tissues such as muscle that was found that type 2 signals, immune signals were important. I'll be talking about that first. Type 2 signals being characterized by interleukin-4. An immune response is not always bad, and this was a little bit different for our thinking of biomaterials. Early on by materials are supposed to hide and be stealth and then when we started looking at putting stem cells in there or promoting tissue repair, we wanted to have a positive interaction with the surrounding tissue and cells.
Here finally, we want the immune system to recognize what we're doing here, but we need to be specific about how it recognizes what's going on. Before we get into that, I did want to review quickly some of the basic immune classes that I'll be talking about. There's the innate and the adaptive immune system and different cell types in each.
The innate immune system is a fast responder, but generally less specific. Cell types that are going to be important here are neutrophils and macrophages and then there's the adaptive immune system, which might take a little bit longer, but it's very specific. You'll have antigen presenting cells that are presenting signals and antigens to things like T-cells and B-cells. We'll be talking a lot about T-cells and maybe another time about B-cells.
Then there's some interesting cells that live on the line between the 2 innate lymphocytes. As the name suggests, they have innate behaviors but they derived from lymphocyte lineage. Then, there's the Gamma Delta T cells, which I won't talk about too much today, but we've published some interesting things on Gamma Delta T cells in the foreign body response.
All these different cell types are not only there, they're communicating and working together. By the end of the presentation, I hope I'll be able to get across to how all these cells are communicating and working together to make a tissue either a healing or non-healing environment. In addition to the cell types, there are certain phenotypes. I mentioned Type 2 immune response. This is characterized by production of the cytokine, interleukin 4, and others such as IL5 and 13. They can be made by number of cell types: eosinophils, innate lymphocytes, and T cells that are expressing IL4, or are called Th2 T cells.
Now, I always like looking at what these normal classical function of these cells are. These cells were associated with host defense, like most cell in the immune system, but in particular, parasites, and helminths, and extracellular bugs. It's also associated with allergy and asthma, some negative sequelae of that to host defense. Then, finally, I'll show you some evidence and there's also much published literature on the importance of these cells in tissue repair, in particular, the liver and the muscle. Another group that I'll be talking about are Type 17 or Type 3 immune responses characterized by the production of IL17 in different forms of it, a and f. These are made by Gamma Delta cells, again,
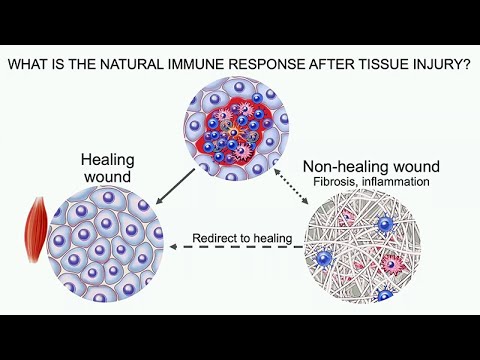
the innate lymphocytes and T cells expressing IL17, or are called Th17 cells. Again, these are associated with certain types of host defense. Then, negative sequelae of this pathway is autoimmunity, so Type IL17 is associated with a number of autoimmune diseases. Also, fibrosis, that makes sense with the foreign body response that this would be important. Let's get back to tissue repair now. As we were shifting to try to understand the immune system and how we might want to therapeutically target it to promote tissue repair, we first wanted to look at what is the natural immune response after a tissue injury, and what does it look like in a healing wound, and how is that similar or different to a non-healing wound characterized by fibrosis and chronic inflammation, and can we use this information to shift that non-healing wound resolve things and redirect it to tissue healing.
The modeled tissues that I'll be talking about today are muscle tissue. We're constantly damaging our muscle if, for example, we're exercising. Of course, if you get a very large defect, you're going to need extra help to fix it. But, in general, is that little damage to the muscle tissue can be repaired. That's opposed to cartilage that I mentioned before. The reason why it was an important tissue-enduring target, it doesn't have much capacity for repair.
Now, there are some biomaterials that can help model or even exaggerate these environments. I'll be talking about biological scaffolds, some of which are used clinically, and then synthetic materials, I won't talk to you about today. We're just going to talk more about the cartilage environment. In order to understand these responses, we want to really map the responses here, defining tissue-specific injury and biomaterial response when we're using that as a model. We want to understand who was there, what are they doing, and who's talking to who.
What I'll show you, then, is you get the same cell types there, but the way they're talking to each other is different and that results in a different outcome in the repair process. We've taken these cell types individually and isolated them and then also looked at them altogether, trying to characterize who's there. We have these tissue maps, it will be useful for designing therapies, targeting therapies, but also relevant to a number of tissue pathologies broadly. We'll be using a variety of techniques. The flow cytometry, standard immunology, for many years is just growing and growing and growing. The number of cell markers that you can look at are just constantly increasing and, of course, single-cell technologies are an exciting area.
Then, even some traditional areas, such as bulk sequencing, sorting and sequencing specific cell types, can be very helpful for rare populations and just confirming results. Starting off at the muscle wound, Caitlin Sadler, who's now at NIH, started putting biological scaffolds into these materials. Biological scaffolds had already been shown to operate by an immunological aspect, so macrophages were important for the therapeutic response.
These are also used clinically and considered to be pro-regenerative. We implanted a scaffold in quadricep defects. You take a punch in there and take a large amount of the quadriceps out, and what we can see is an increase in interleukin 4 productions. I mentioned interleukin 4 was important for tissue repair and we could increase that with the scaffold in there. Now, you might have a question about scaffold composition.
We did evaluate a number of different sources of that tissue extracellular matrix. You can use small intestinal submucosa, bladder, urinary bladder matrix, bone matrix, even cardiac tissue matrix. You can pretty much get the same biological outcomes depending on the source.
You might just process them differently for different applications, whether you want particulates, or powder, or sheet. If we implant these biological scaffolds, if we do, first, a characterization of the myeloid populations, the labels here are down with the different colors but you can just broadly look, there are different cell types there. The muscle injury alone, we've got those cell types. A lot of macrophages, we have different ways to characterize macrophages. Then, we have the scaffold in there. First of all, we have a lot of eosinophils but, then, also the scaffold-associated macrophages that are a little bit different than your standard macrophages.
Now, if we look at the standard markers for macrophages, these are rather classical, the 86 pro-inflammatory and surface macrophage 206 being more pro-regenerative or alternatively activated macrophages. The scaffold decreases the pro-inflammatory macrophage marker expression on the surface of cells. It doesn't change too much with 206 except for one scaffold that induce more fibrosis. But, here, I want you to look at the field of pod, this is 206 and 86, the majority of the cell types, that macrophages, express both. They're present together; it's just the ratio of one to another.
I'll talk later about how we've used single cell to get better surface markers to characterize, really, what's happening with macrophages. Now, let's look at the adaptive immune response. We notice that CD4 and T cells are increasing.
CD4 T cells are considered the T-helper cells that secrete cytokines, including interleukin 4. If we look at the wound alone, which is treated just with saline water versus the wound with the ECM biological scaffold, early on, we get interferon gamma and also IL 17 being expressed by the CD4 T cells. But IL-4 then, tends to take over and increase with time. Again, moving things towards resolution and regeneration.
Now, if we look at that IL-4 little bit more, this is with a mouse that has to be connected to IL-4. Again, we can see you have a little bit in the wound alone, but when you put in that scaffold, it essentially gives a power boost to that TH2 T cells. You get more of them and you get more expression of the interleukin 4. Now, I showed you already what happened to macrophages. Now, if we do not have T cells there, so if we take away those T cells, those macrophages shown here in red then in the Rag knockout animal increased significantly their expression of that pro-inflammatory macrophage cell surface marker and we lose the CD206, so double-whammy, more inflammation and less resolution.
Now Kayla, notice something very interesting. She picked up that draining lymph node and notice that when there was an implant, it was larger and you also got interleukin 4 expression there. This was particularly interesting because of two things. Number one, it means that the local injury, the local implant, could be having systemic effects, regional and systemic effects. Then also introduces the possibility that systemic aspects of the organism can impact the local response to whatever is going on about the wound or material. Now, to probe a little bit more that T cell response, Jonathan Powell had Richter knockout mice and we can take the Rag knockout mouse and put either normal T cells in or T cells that were deficient in the Richter protein, which is a part of the TORC2 complex, which is critical for TH2 differentiation.
The T cells and those mice could not differentiate. What we found is you can see there's additional fibrosis and epigenesis instead of the nice repair tissue. Those T cells were important in directing those macrophages, but it also again, makes the point that the innate and adaptive immune system are working together, talking to each other. Back to looking at some some cell types. We have been exploring single-cell techniques. I mentioned the macrophage diversity.
We pull out macrophages and run a single cell and we essentially have a better definition now of different macrophage subsets, ones that are more fibrotic and ones that are more regenerative. We've also done that with fibroblasts, so there's a lot of exciting work going on in fibroblasts, but we're particularly interested in the immunological rule. We have lots of different cells talking to each other in not just the traditional immune cells, but also a tissue resident fibroblast.
We have a macrophage data set here. We have fibroblasts data set here and we also took CD45 enriched single-celled data sets. A lot of different cell types here.
We first ran the single cell and you can see that there are many different clusters, many different cell types, and when we compare it to the cluster composition between groups, these different treatment groups, whether it be the biological scaffold, the wound or no injury, we don't see huge differences. We don't see the same differences we would expect based on the different biomaterial implants, so we looked at building a program, Chris Cherry built a program that does an estimate of how cells are talking to each other. There are a few programs out there, but he went about it in a different way. Looking first at transcription factor activation and then looking at how it correlated with certain receptors that we're signaling. This is important because a lot of times, we don't catch the expression of ligands. For example, eosinophils making IL-4 are going to be hard to capture with single cell, but we can capture the receptor and the activation of pathways associated with IL-4 and the transcription factor activation.
This Domino program does this an unbiased way. It's independent of the clusters that you normally see with single-celled data sets. This is what it looks like when we have a pro regenerative muscle environment versus a fibrotic muscle environment. You can see the communication pathways are pretty different and then we can look at what are the specific transcription factor activations in a healing versus a non-healing wound.
This is what it looks like. Now, what's interesting is that you have an immune module into specific modules. In tissue specific modules where I would put things like stem cells and then we have a fibroblast module. The way they communicate is different and we have some particular predictor receptors and transcription factor activation that you can look at and then probe further using this program.
This is going to be important when we look at that non-healing wound. In particular, looking at those non-immune cells that are immunologically active. When we're trying to make cartilage, I showed you data that, a focal cartilage defect, which is generally more of an athletic or trauma injury and if you're in a healthy environment, you can get some decent repair. But most of the patients who need cartilage repair have more of a diffuse degeneration connected with chronic inflammation, so you do have an immune component. What we found is when we put these cells from an arthritic environment or expose them to cytokines like interleukin-1 Beta, you decrease tissue production, so that main part was important. At the same time, we found in collaboration with unity that senescent cells are present in no way, so that's been published for a while and we looked at what is the real active component of these cells and are they positive in arthritis? What is a senescence up? Senescence was first discovered by Hayflick in the context of replicative senescence, and then later telomere reduction and sort wearing out is another area that is important to senescence as the cells get older and can't proliferate anymore, the telomeres reduce, then you also have stress-induced or pure premature senescence.
Oxidative stress, oncogenic stress and what I'll show you, immunological stress can induce senescence. Just like the immune system, sometimes they're good, sometimes they're bad. Judith Campisi showed how senescent cells are important for wound healing. She characterized that fibroblasts and endothelial cells where the primary senescent cells there and you needed them to get efficient repair.
Then I'll talk to you about how chronic senescence or having senescent cells around too long that don't get cleared actually inhibit regeneration. What are senescent cells and how do they operate? They are in proliferate arrests, so they are no longer dividing, but they are far from quiescent. They're actually quite active and they secrete a senescence associated secretary phenotype or SASP. This SASP is implicated in their pathologies in promoting tumors or various age-related diseases such as heart disease, of course, arthritis and diabetes. But then as I mentioned, there are positive factors in tissue repair. We first use a mouse model developed by Judy where you had a p16 promoter connected to something that allows us to visualize the cells, but then also a kill switch with ganciclovir so we could selectively clear this p16 positive cells.
When we made a joint injury and a joint injury, Sham just opening up the joint. Very mild injury versus an ACL transection and this is vehicle. You can see an increase in senescence cells or the bioluminescence at least.
Then if we give ganciclovir and kill those senescent cells, we can get rid of them. This is a nice model to see well, that these senescent cells just correlating with disease, or are they causative? If we use that genetic model or some drugs, what we notice is that when we clear senescent cells after that injury, we significantly decrease that inflammation. You can look at a number of factors that are suspected SASP factors, interleukin-1 Beta, interleukin-6, a lot of MMPs. Then you can also look at functional outcomes such as pain.
If you remove those senescent cells, not only do you reduce inflammation, but you also reduce pain. Then surprisingly, we saw that when you declare those senescence cells, you got better tissue repair. You can get resolutions. This I think is important because we're not keeping any growth factors, we're not giving any stem cells. We're just removing inhibitory factors that are blocking the tissue repair process. Ultimately clear senescent cells and various sense factors and you can reduce the senescence associated secretary phenotype, which includes a lot of inflammatory factors, reduce pain and increase tissue repair.
We were quite interested in some of these SASP factors such as IL-6 and IL-1 Beta. Because as we look at how things might be communicating, these factors are actually known to promote the immune cell differentiation down particular pathways. In particular IL-6, IL-1 Beta in the presence of TGF-Beta induces a type three or that interleukin 17 mediated immune response. We went back to the joints and looked for that and what we found was in particular CD4 and gamma/delta expression of IL-17 ANF increased significantly with that ACL injury. IL-17 was also secreted by things like innate lymphocytes. A number of different cell types are all making that IL-17.
Now that's a trauma. Osteoarthritis is considered a local disease. Just wear and tear and the joints wearing out.
But just like the muscle where I said we'd looked at the draining lymph node. We also looked at the draining lymph node here. What we found was really significant increase in both the general gene expression and also specific cell types. This is the CD4 T-cells making IL-17 or TH-17 cells increasing significantly in the lymph node.
Particularly in the joint which doesn't have a lot of vascularity. You don't have too many immune cells there. That draining lymph node provided almost a magnification of what was going on. We can really see these immunological changes very nicely.
What happens with a senolytic? Again, it's making that connection between the senescent cells, fibroblasts and the IL-17 immune cells. What we found is when we delivered that senolytic, This is looking in the lymph node, you can significantly reduce those signatures of IL-17. This is very exciting because it's a way that we can make a connection between the two. If we neutralize IL-17, we can decrease expression of factors connected with senescence.
In particular p16, and this is p21. We went in vitro to validate this little bit more, and this is what we found. If we artificially induce senescence using radiation and expose those cells in vitro to naïve T cells, activate those T cells and put it in the presence of TGF-Beta, we get a significant increase in IL-17. Now vice versa, if we take Th-17 cells and co-culture them next to healthy fibroblasts, we can induce senescence in those fibroblasts as seen by number of SASP factors that increase in p16.
If you look at the expression profile of these cells, it's quite different. This inflammatory induced senescence has different characteristics compared to the standard classical ways of looking at senescence with oxidative damage. One important factor though, is, can we still repair when old? Many people coming in with that joint degeneration are not, 10 week, 12 week mice. Actually reviewers ask us this first and Jan Van Deursen published a paper using another mouse model clearing senescent cells and looking across lifespan.
I asked him, do you have any joints left? He didn't look at the joints and thankfully a few joints left. What we saw was amazing. When you clear the senescent cells here is beautiful cartilage, and without that, you can barely see where that joint space was.
Now, if we looked at the senolytic just in wild-type animals, you first you see before surgery those joints are not looking so great and the injury makes it look even worse. Senolytic doesn't really do too much. You can decrease inflammatory markers, but you don't see much tissue repair. Now another collaborator, Daohong Zhou, has been focusing on the bone marrow and understanding senescence in the bone marrow and how you can rejuvenating the bone marrow.
One thing we notice in the old animals, if you'd look at the CD4 T cells in the lymph node. They're not so many of them. You really don't have too many T-cells left in the lymph node as you age. We've looked at using the local senolytic like we did with the young animals.
Then also the systemic senolytic that Daohong used to rejuvenate the bone marrow. This is what we saw. Again IL-17 going down with senolytic in the young animals. But in the old animals, we can also get IL-17 going down. Not a big deal. But what was different was interleukin four, that same cytokine that was important for muscle. When we gave that combined inter articular and systemic senolytic, that was the only time we got an increase in interleukin four in the joint and old animals and saw a nice cartilage repair.
You can repair when old, but you probably going to need some help. You're going to need some additional senolytic or additional addressing of the systemic immunological changes, which also you finally in muscle. This was in clinical testing by Unity. They tested, phase one looked good and they saw a nice correlation actually in phase zero between senescence markers and severity of disease.
Phase one and phase two. But a little tricky part here. They did a single injection and patients went up to 85 years old. What I didn't tell you in our regimen was we did multiple daily injections and you actually needed at least three injections every other day so that dose really was important. Then I showed you the aging. P16 in older animals represented here by a is a lot higher.
The dosing matters and then the patient population. This bring some more questions and can regenerative therapies still work in aging? We did the cartilage work just came out last year and now we've got some work in the muscle, which I'll post as a pre-print hopefully pretty soon. But this is exciting because it's quite intrigued by this paper from a number of years ago that with aging, the muscle stem cell population is still there was not really functioning. But if you take it out and restore it, it can actually function again. The stem cells were there, but there are things just blocking them. Again, thinking of the inhibitory factors of senescence, what are all these things that are blocking tissue repair? Here's that same muscle injury model, the same biological scaffolds.
What happens in an older animals? Well, I know there's a lot of data here, but the main factors are we saw with aging eosinophils and CD4 T cells decrease. This is high parametric flow cytometry looking at young and old animals. Here if we pick out a few pieces here, so these are the CD4 T cells increase. I showed you that in young animals, not the same increase in old animals. On the other hand, CD8 cells increase. CD8 cells are the ones secreting usually pro-inflammatory factors.
A reduction of the helper cells, an increase in the pro-inflammatory cells and then our eosinophil numbers going down. The CD4 T cells and eosinophils were important in making IL-4. We did single-cell again and just like I showed you with dominant, we didn't have a large number of samples per group. It's pretty expensive.
If you just look at differential expression in clusters between the young and the old, you don't see much, but we can use techniques such as this non-negative matrix factorization and we found that markers of collagen are higher in the old, some more fibrosis related things in the old and in the younger animals you have more macrophage activation in antigen presentation. Then when we apply domino, so that cell interaction, we see some really interesting differences. Again, these are the different modules talking to each other. This is unbiased and you just group together.
The cell types that seemed to be interacting together. You have your immune tissue, fibroblasts and then antigen presenting module. Lots of connection here in the young animals. But look, we're losing connection in the old animals, in particular, this fibroblast module is really off on its own.
We're really dysfunctional in some cell communication. Jin Han, who worked on this, looked at some string analysis to look at protein-protein interactions, to see what was going on and is predicted that all 17 signaling increased only with injury and the aging environment. This was pretty mean because if you just compare the young and the old with these various cytokines that were predicted with this analysis, you don't see a difference.
It was only after that injury that the old animals had this crazy response. We looked at this a little bit further. Again, looking at the interleukin 4 and IL 17. IL 17f in the muscle tissue is another example of one of the factors that only went up with treatment. Gamma Delta T cells were an important component that was making IL 17.
If we look at just general gene expression, you see all IL 17f. If we do flow cytometry and staining for IL 17a, you can see it in the Gamma Delta is increasing. Oh did I forget the most important key part? [LAUGHTER] Sorry, I missed one slide. This is new data that just came out.
What we can do then is actually give neutralizing antibody to IL 17 and recover some of that therapeutic response. Really this type of analysis gives you new therapeutic targets to look at. I really believe that we will be needing combination therapies. We've got all these different cell types, communicating, working together. You've got environments changing such as the aged environment. There's no way a single therapy is going to work in both the young and the old.
We're going to need these combination therapies, whether it be some analytics, systemic, and local or an immune factor along with the biological scaffold. What does this concept of regenerative immunology. We are connecting together regenerative medicine, immunology in tissue engineering. But there's still a lot to do to map this immune response to injury.
People think the innate response for a pretty good amount of time, but the adaptive response is quite interesting and we're now pursuing antigen specificity. Even a memory of his injuries and even biomaterial implantations and a lot of clinical samples coming from that. A lot to do for mapping, these immune responses.
Then understanding how does immune environments impact downstream tissue repair, including things like stem cell activation, cell types like fibroblasts and senescence, how it might affect vascularization, things like that. Then use this information to engineer immunotherapies to create a pro-regenerative environments. Whether it be a self therapy, neutralizing antibodies or small molecules analytics or biological scaffolds, we can use the information from this mapping and communication in the different tissue environments to promote tissue repair.
My cancer immunology collaboratives like to say that the immune system is therapeutically accessible, so it makes it a nice targets for regenerative medicine. Many people to thank lab members, both current and alumni involved in this work, collaborators at the Bloomberg Kimmel Institute for Cancer Immunotherapy. In our computational collaborators as we've moved into the single-space senescence, collaborators and of course, our clinical collaborators who are helping us with clinical samples. With that, thank you for the invitation to speak today and thank you for your time. That was terrific, Jennifer, thanks so much and very stimulating. If anybody has any questions, please just put it into the Q&A at the bottom of your screen.
They're starting to come in. Interestingly, there are a mix of both very specific and also very philosophical. Maybe I'll alternate between them.
[LAUGHTER] The first question is, CD4 T-cells, do they make immune synapses with any of the macrophages? Is the CD4 reactions specific to any particular antigen that might be presented? Fantastic questions. One, I didn't mention what the scaffold associated macrophages look like. When I showed the myeloid reactions to those scaffolds, I have those dark blue scaffold associated macrophages. Those are co-expressing CD11b and CD11c and our MHC to high. It does suggest they are doing some antigen presentation.
I think it does make sense, particularly if you think of tissue damage and all the pieces that need to be cleaned up, tissue damage that there would be some antigen presentation. Actually we also do see some tertiary lymphoid structures. Where you have macrophages, T-cells, and B-cells. You see those tertiary lymphoid structures.
Your last question is the antigen-specific T-cells. Two pieces. Number 1, we are in the process of doing TCR analysis with single-cell dataset. I will have information on clones next time. Hopefully not too long.
We're pursuing understanding that clonal response. More specific data that we have is in Liam chung's Science Translational Medicine paper. It was specific to the foreign body response, but I think it's relevant for tissue repair. He made a bone marrow chimera of wild-type bone marrow and marrow from an OT-II mouse. That OT-II mouse can only respond to ovalbumin. We're were curious, would you see that same increase in IL 17 in this case associated with fibrosis, foreign body response around the material.
Essentially, your wild-type mice increased, there are 17 production in CD4 T-cells. Then in the OT-II mice, you do not see any upregulation in IL 17. There's no ovalbumin there, so does not appear to be any non-specific activation of those T-cells. I do think it is going to be antigen specific and we will have TCR clones and we're trying to figure it out.
I'm excited. Ecited, great. Thank you. Some of the questions are actually skewing a bit more philosophical but- [LAUGHTER]. I'm curious what a philosophical question could be. [LAUGHTER]. It's meta.
Given that the initial reaction clinically to most injuries, particularly in the musculoskeletal system, is to give anti-inflammatory agents and said steroids, things of that sort. Your findings that inflammation is genus face. It does some good things and some bad things. What are your feeling about? What standard of care is most of the time for orthopedist? That's a great question. I think, Steve Battle Lake, did publish that looking at the response to a biological scaffold and incense suggesting that you would get some reduction in the therapeutic response at that biomaterial with the enzymes.
However, with regular injuries, I think it's a good question. I think there should be some data out there on that published at least from the physical therapy exercise training perspective. But if you think though about the joint where it's not a good immune response. You've got not at the cell types that you generally want. You can imagine an anti-inflammatory would be helpful.
We did do an experiment in the cartilage of a steroid injection in addition to the sentalitic. What we did notice is that it did block all the immune cells, even the ones that were making interleukin 10, which increases for the sham surgery and seems to promote repair. I think that is consistent also with clinical data that steroids tend to lead to further joint degeneration. Yes, I think we need to be smarter about our inflammatory targeting. Great. First of all, complimented on terrific work, which I agree.
Thank you. [LAUGHTER] Did you expect almost complete reduction in interleukins with the removal of this senescent cells, aren't there other cell types contributing to interleukin levels? Absolutely. This is just a snapshot in time. I think this analytic is going to be temporary, first of all. If we look further along after this analytic treatment, await further time points, You see the increased joint degeneration.
Yes, there are other cell types besides T-cells. We're also looking at co-culture of senescent cells and macrophages, lots going on there. A lot of times when you clear out a T-cell, innate lymphocytes can compensate for the cytokine production. We did show that innate lymphocytes are making that.
You might have a temporary reduction in those cytokines and it's not completely zero, the cytokines. But I think the presence of those cytokines are going to induce more senescence. It's a positive feed-forward.
You need to address both that stromal and inflammatory cell or senescence cell in addition to the immune inflammatory cell. Another argument for a combination therapies, which I think presents a unique challenge from the clinical trial perspective and regulatory perspective because combination therapies are going to be challenging. [LAUGHTER] I certainly agree in your last comment, almost identical to the end of my talks as well. [LAUGHTER] The regulatory hurdle that combine therapy have to- The next question actually starts dealing with the difference between your mouse models and actual human systems. There are significant variations in the Gamma Delta T cell populations between mice and humans.
For example, the homotopic receptor dendritic GDT cells in the mouse epidermis has no equivalent in humans. Have your wound repair studies been correlated in humans viz-a-viz your mouse models? Also great presentation. [LAUGHTER] Thank you very much and that's a fantastic question.
I will point you to Liam Chung's Science Translational Medicine paper on IL-17 in senescence in the foreign body response, and there we're able to get a lot of clinical samples from breast implants, or essentially the tissue expanders that are placed before the permanent implant. We get that tissue sample when those are being exchanged, and we can get the cells out of that. There is where we see a lot of the Gamma Delta T cell.
Much more so when we show this data to our immunology collaborators, they're like, "What an incredible number of Gamma Delta cells there." That's just in that tissue around the breast implants, right? Not where you'd expect to have a ton of Gamma Deltas. I think we're going to be finding new function of Gamma Delta cells, and just as I mentioned, we have that TCR analysis from single-cell data sets in the mice coming. We're also doing that with the human samples.
We're going to try to look at the antigen specificity of that in addition to understanding more about the Gamma Delta cells in the clinical samples. Yes, they are very different in the skin. I don't know where they're coming from in this tissue sample. Again, this is a different scenario in the mouse studies.
When we put in a gut infection that induces IL-17 in the gut, we see more Gamma Deltas in the draining lymph node from those implants. I think they potentially act as an environmental sensor, and impacting what's going on in the local tissue in that way. I think it's a super exciting area. They are there in human samples, and we're trying to figure out what they're doing besides making IL-17 A and F. [LAUGHTER] All right, this is one of the more philosophical questions.
Given the use, not just in unregulated clinics, but even in some orthopedic departments, the use of MSC therapy, injections for arthritic joints, and things of that sort, have you formulated, based on your work, an opinion about those? Well, I would say I had an opinion even of chondocs when I was in graduate school. Oh, sorry, that was not the product, Carta cell. The autologous chondrocyte implants, right? What I had always thought somewhere that, we're delivering dead cells.
What's interesting is that there are papers now that are being published, applying them to cardiac injuries, and I think another one came out recently too, that essentially dead MSCs, right? They inject to get those stem cells, a lot of them dying, actually can give you a pro-regenerative immunological response. I talked about tissue damage, and damage associated molecular patterns that can induce immune response. Tons of them get stuck in the spleen, an immunological organ. Not for the intra-articular injections but just the idea that a dead cell can have a therapeutic impact. I'm not making a judgment, I'm just saying that a dead cell, especially if you have millions of them, can have a significant therapeutic impact.
Are you mobilizing more immune cells, changing the immune phenotype? I can imagine many mechanistic scenarios where that could lead to some outcome. How's that? [LAUGHTER] Okay. Perfect. In your experiments when you injected senolytic, presumably that was systemic, did you see any impact on other organs other than the musculoskeletal system, for example, heart or brain, or did you not have an opportunity to look at those organs? For the young animals, we injected the senolytic locally into the joint intra-articular, and then the older animals we did the systemic senolytic navitoclax, that Daohong had published. He focused mostly on the bone marrow and the changes that happen in the bone marrow.
Essentially with aging, the bone marrow becomes more myeloid skewed, so you can look at lymphocyte replenishment in the marrow, and rejuvenation of the marrow, having it look more young per se. Periodically, we have looked at kidney, and liver, and lungs senescence, and usually with these systemic senolytics, they will decrease but we used the same regimen as Daohong, so we didn't spend too much time analyzing that. That said, I think the bone marrow is really important as a place where you have some memory of injury or some aspect. One of my favorite papers recently is Catherine Moore's paper looking at myocardial infarction, and breast tumor growth. That tissue injury in the heart impacting tumor growth in a distal site, and they found that epigenetic changes in the bone marrow, monocytes were in part responsible for that, and it could be transferred to another animal. I just think that's amazing.
I think it's incredible to think that if there's some injury or implant in the body, you can have a memory of that imprinted in your bone marrow. That's philosophical, right? [LAUGHTER] We're getting towards the end of the hour, and the last question is a bit philosophical, so we'll make that the last question. Given that you indicated that senescent cells also seem to be a bit Janus-faced, and that they have, for example, as you pointed out, Judith Campisi talks about their positive impact, I think I know where you're going to come down on this. Is it that let the senescent cells as they become senescence, stop producing good things or do they start producing toxic things? We do have some data on this and we're working to get this paper together.
I think we've started to find the good and the bad senescent cell. We've done that using a transfer learning technique where we can get bulk signatures of a senescent cell by sorting out specific populations from a transgenic that allows us to label them bright enough. We can do the full cytometry to understand which cell types are senescent and then sort them out specifically and do bulk sequencing. Take that bulk sequencing signature and apply it to single cell to understand which clusters are most similar to the senescent cells because you can't capture senescence in single-cell for a variety of reasons that I don't have time to get into, but it's hard to see them there.
We have some clusters that we think are associated both with looking at the signatures of those clusters and then fish to see where they are. We think some of them are associated specifically with angiogenesis, and some of the senescent cells with a different phenotype, actually they have a cartilage-like phenotype, but they're in the area of fibrosis. I think there are different types of senescent cells, and we are going to learn which ones are good and bad.
There's probably a kinetics, right? How long they stick around and if they're reversible or being cleared. But I think there are different ones, and we will be defining the good and the bad, then we can develop really good drugs. Keep the good ones but get rid of the bad ones.
That's the dream. [LAUGHTER] Anyway, one last breaking question came in. It's not so philosophical. We'll end maybe on that one.
Okay. Fantastic presentation, and could you please comment on the potential impact of extracellular matrix on stem cell senescence in vitro. The extracellular matrix scaffolds on stem cell senescence? Probably. I suppose you can brighten it. Is there any relevance to the substrate that's, they are either the grown on or what you might encounter in situ.
Yeah. In vitro you don't have the immune aspect. Then you are depending on specific factors in the ECM, whether they be as Steve Butler talks about the Matrix bound vesicles that have important factors in them, or certain ECM components that can help proliferation. But what I think is interesting also is potentially in the cultures clearing the senescent cells as you move forward, because essentially those senescent cells that are left there can spread senescence or cause trouble with the other cells, and it's a domino effect. If you can be culturing them and as you go work to clear those senescent cells, I think you'll be able to keep your healthy ones healthier longer. That's a great answer.
It gets into a whole other territory [LAUGHTER] of the senile brain that we don't have time to get into. Thank you so much. We've come to the end of the hour, in fact, we've gone over, you've been very generous if you look at the time, and it was a terrific, and stimulating presentation. Thanks so much. Thank you so much. Have a good rest of your day. Bye bye. Thank you very much. [MUSIC]
2021-08-22 11:49


