TRACO 2017: Non-SCLC and HSP90 chaperone
Okay. We're going to get started and our first speaker today is Eva, Szabo she's. Chief of the lung and upper aerodigestive, Cancer. Research Group at. The, NCI, division, of cancer prevention. She. Graduated. From Yale University and. Got. Her MD at, Duke University, she. Did her internal, medicine, residency, at Bellevue New York Medical, Center and. After. Completing a medical, oncology, fellowship, she, joined NCI. And. I've known her for many years, I'm sure you'll enjoy your lecture on non-small-cell. Lung cancer. Eva. Thank. You teri everybody. Can hear me is this. All. Two, of you no sorry, thank. You for being here in person and, thank you all of you online I was telling teri that this gets weirder and weirder as you're, talking to an empty, room but, I've been told there are others listening, in so so. We're gonna talk about non-small-cell lung cancer, I think you've already had the small cell lecture, and. I'm, gonna spend actually a fair amount of time about prevention, which is my day, job so to speak so. A little bit about lung cancer statistics. I've. Been doing this for many, years the many years that I've known teri, and, the. Number of new cases still continues, to climb it's over. 220,000. A year and. Almost. As many deaths for, this year the estimate, is about 155. 56,000. Now, there's no question that lung cancer is the leading cause, of cancer, deaths it's greater than the next, three causes of. Cancer deaths put together however. The, good news is, that the, death rate has, been decreasing, as we're decreasing tobacco, smoking. And. Even in women, you. All are too young but when I was, a. Youngster, the. Tobacco. Ads. Where you've come a long way baby Virginia. Slims. Celebrating. That women have broken through can smoke just as much as men and then, there you go now lung cancer incidence went way up it's, now finally decreasing. Because. Lung cancer is a very, long, the, lung is a very deep internal, organ. You. Don't find out that you have the disease until, it's fairly advanced, so the five-year survival is, quite poor 16, percent 16. 18 percent altogether just. A minor increase. From, 5 to 16. Percent over. The past 60, plus years. So. A huge. Problem, now. The risk factors are well known. Yeah. Here we go 85, percent or so is due to tobacco that includes, passive. Smoking so if you grow up in a family where, there is a lot of smoke or your spouse smokes, that's, bad. It's. A field, effect so, if you've had a prior aerodigestive. Malignancy. Had in that cancer for instance you're, at increased risk of having a secondary, lung cancer, chronic. Obstructive, pulmonary, disease COPD. But. Here's. Really the picture, so this is a picture of somebody with a lung, cancer, here's. All the fibrotic changes that. Are and, it's. Hard to see but this is some, COPD, there and there's. The pack of cigarettes okay. And there you have lung. Cancer in, a nutshell there. Are other exposures. That are also important. Asbestos well-known.
Obviously, Women trying to get rid of that over, the many. Years indoor. Radon we, check our houses right our basements, when we're, lucky enough to buy a house and then a number of. Exposures. That people get as part of their jobs. Chromium. Nickel etc. From various, industries. Inorganic. Arsenic, which is in groundwater not. So much in most, parts. Of the US but in other parts of the world. Genetics. Has been a little bit hard to study because. When. You live with smokers you also get their genes if you're a child. But. Nevertheless there, are some genetic predispositions. Very. Rare germline. Mutations. In the, epidermal. Growth factor receptor. Gene. The, t7 90m, mutation. And I'll be talking about this a little bit because this is an important, target. For, cancer. Treatment. There are various. Nicotinic. Acetylcholine receptor. Subunits, that are associated with. Increased, lung cancer risk and interestingly. Enough. One. Of these is also a susceptibility, locus, for COPD, helping. To put, this together. Why COPD. Is a risk, factor one, of the reasons why, COPD, may be a risk factor for lung cancer this. Is by no means an all-inclusive. Overview. It's I'm, just giving you sort of the highlights. So. Now small cell lung cancer, is is. A basket, term, it. Really includes several different, histologies the, most important, one being AD know carcinoma. And. It's. Got specific. Patterns. Of how it looks under the microscope these, tend to be peripheral. Nodules. That obviously. Spread, and this, is the most common, type of non-small-cell lung cancer, and. It includes the, non-smoking. Associated. Lung cancer but, most adenocarcinomas. Are still due to tobacco, exposure, about. 20%, of squamous cells these tend to be more central, tumors different, appearance under the microscope, and. More. Heavily associated. With, tobacco exposure. Somewhat. Less common are, the large cells which also tend to be more peripheral. And not, as clearly associated, with. Tobacco and then a smattering of, other rare, types, of histology. But. We tend at least in the past we, tended to lump everything together it is not the. Small cell that, you learned about a few weeks ago and here's. Of course small cell you learned about that, so. Long. The development, of lung cancer, is not is a it's, a process, and it's a process that therefore, allows, us many. Opportunities. For intervention, although. The. Bulk of what we do in medical, oncology, is, right here early, to late cancer. Once we find it we cut it out we, radiate, it we, give various. Systemic. Treatments, I, will be talking about the, much longer phase, from, the time of initiation where. The exposures. To tobacco, for instance come in and, damage, the respiratory and, alveolar. Epithelium. Through, the gradual. Changes, dysplastic. Changes. Until, you have the development of a locally. Invasive, and ultimately metastatic, cancer. So. The treatment strategies, for lung cancer are very similar, to other types of cancers they're very anatomically. Based, early. Stage at surgery, you, take it out if, you have more. Local. Involvement. Including some lymph nodes, either. If you you can if you're if, you can still resect, then, you generally follow up with adjuvant chemotherapy, and, that has been actually shown to, improve survival. So. That's standard, for stage 2 and stage 3 a, once. You get past to, just the very localized, tumors. And you start to have lymph nodes in the mediastinum. Then. You're generally talking about combined modality, therapy, so that's usually, chemo radiation and, sometimes, you, even add surgery. To, that but. Once you get to the more. Extended. Involvement. Either what. Used to be called a 3b wet in other words a. Pleural. Effusion or. System, which is really systemic, spread, or. Any, kind of distant, metastatic. Disease then. You're talking about systemic. Therapy. You. Can use radiation as needed for local control sometimes. You will if you only have one isolated, metastasis, for instance in the brain which, is a privileged site you may cut it out but, for the most part you talking about systemic, treatment, of course that's the world that I live.
In As a medical oncologist and, small cell lung cancer, you heard about, so. What's changed, is. That. We now understand, a lot more about the, genesis, of various. Types of, lung. Cancers, and we now pay attention, to the, histology as, well so. It turns out that there are a number of, different driver, mutations. That, are present in, aDNA carcinomas. And in, fact we can ascribe. Various. Drivers to, about. Two-thirds. Of all, adenocarcinomas. And for, some of them that, not. That one for, some of them they actually serve as a. As. A basis, for targeted. Therapy, treatment, and so, this really started. Out with the epidermal, growth factor receptor. Which. Is a, very, important, component. In. Cell. Signaling, and. Turns. Out that about 10 to 15% of. ADNA. Carcinomas. Have. EGFR. Mutations, and if. You have an EGFR mutation. Then, there are various. Drugs, or lot nib a fat nib to fit nope Oh a. Series, of drugs that will, give, you a very, hard these are oral, tyrosine. Kinase inhibitors, and. They interfere. With EGFR, signaling, and they are, associated, with very. Substantial. And prolonged. Response. Rates so, in contrast, to chemo which about 20. To 30. Percent of people will, respond, to and usually, for, about five or six months if you, have an EGFR mutation. The, response rate is, three. Times as large and. PFS. Progression-free, survival is. Doubled, okay, and. Median survival, is also, about doubled, this, is a minority of people okay. But, this is a very important, treatment. And this is standard of care if you have somebody with advanced, non-small-cell, lung cancer, you will check especially. At no carcinomas, you will check for EGFR. Mutations. We. Now have know. A lot more about the mechanisms of resistance we. Have second-line. Drugs OC. Merck nerve is one, that deals with a very specific, mutation. That, in the EGFR, receptor ajf. Receptor. Which, occurs, in about 50. People, who progress after these, first-line drugs. It's. 37 IBM mutation. And again, you can get extended, progression-free. Survival, although. Ultimately everybody. Does progress and die and interestingly. Enough, this t7. 9dm mutation, is the one that I mentioned earlier, which is a. Familial. Form of. Non-small. Cell lung cancer. There. Are other. Molecular. Abnormalities. Which, are very important, and which we check for. When. Somebody has stage 4 disease the. EML for alch fusion, gene is. Occurs. In about 5% of.
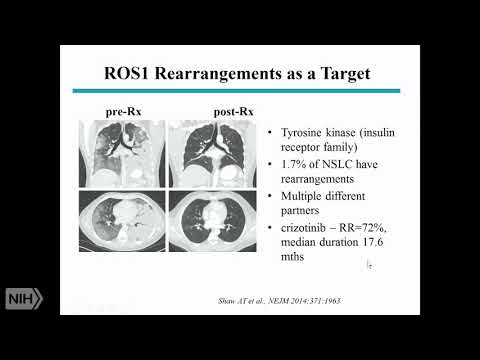
Non-small. Cell lung cancers again mainly adenocarcinoma and, just, like EGFR, mainly. In never smokers ok. And. There. Are now three. Four, drugs, that, are actually. Approved. For treatment, very. Similar, to the EGFR, story, about, 60%, 50 to 60 percent response. Rate and. About. 8 to 14, months duration, of response so. If you are unlucky, enough, to, have. Lung cancer but. Lucky enough to have one of these molecular. Abnormalities. Your prognosis is much better there's, more. Options and of course after progressing. On these various, oral, tyrosine. Kinase inhibitors. Chemotherapy. Still, remains an option, Ross. Swan is another. Abnormality. Similar. Story, perhaps better, duration. Of response using. Crizotinib. Which is the same drug that we use for elk. Translocations. B-raf. FDA. Recently approved. It. This is much less common about, 2%, have, the v600e. Mutations. And be raf citizen. Of 2%. Of non-small, cells this. Is the same mutation you find in melanomas. And this is the same regimen. That, gives, very. Similar. Melanoma, and it not small so Rhett, met, 4x on skating I'm just the, reason I'm really putting this on is to show you that these, are now all important. Because they serve as a basis for treatment, but, rare, mutations. And so you can imagine the complexity, because. How do you know that somebody has a mutation you have that issue and so, now we. Get tissue on everybody. And we. Send it for a molecular, analysis. And. It's. Expensive and difficult and, some people you can't get enough tissue so, you can understand, that, the. Science. Of how we are, the. Arch of how we do treatment. For lung cancer has changed, and it's, complex. When. It comes to squamous, cell we don't have the same option, so all of these things, that I showed are primarily, in aDNA, but not exclusively, and adenocarcinoma, x' you. Know there fgo, the fibroblast, growth factor receptor. One amplifications. Which. There. Are inhibitors, in development, but since 2010. Nothing has come on the market so, this is not the same story. Of driver, mutations, that you can target. This. Is another abnormality. Again now in a very small percentage, of squamous cell carcinomas.
In Vitro, we have drug ii, treated, but, since 2011, this has not come on the market, so therefore. This. Has not been as. Targetable. As what, i. Described. In add no carcinomas. What. Is even. More, new if i can put it that way, then. Targeted. Therapy, and non, small cell lung cancer, is the poster child for targeted therapy it's also the poster child for immunotherapy. And that's what you see in the news, every. Day the. Program def. Ligand. This. Is this, all has to do with t-cell. Activation. I am, NOT going to do justice, to, discussing, immunotherapy. But, the bottom line is that. The. Goal here is, to undo. The. T-cell, limitation. Of activity, or, t-cell active in activation. And to reactivate, the t-cells which are often, blocked. In. The, setting of cancer, and so, it's a ligand. This is just the beginning of immunotherapy the. Ligand, for this receptor, which. Inhibits, t-cell activation is, PDL, one and there, are now a number of antibodies. Approved, some, of which are target. PD, one the receptor, vs PDL, one the, ligand and so, here's just an example of why. This is so exciting so we, now have, approvals. For both second line treatment, and frontline. Treatment, with. Several. Different antibodies. Three. For second line one. For frontline and the. Reason, that, this is really key is if you look here, is that, there seems to, be a, plateau. To, this survival, curve so. People who respond, tend. To respond for a long time sometimes, for, years. And. That is a, game-changer, for anybody, who's ever treated. Lung. Cancer patients, with chemotherapy or even targeted, therapy, they, will stop responding, and usually, that's less than a year but, some of these responses, have been extremely. Long term and so, people. Are very. Excited. About. The. Possibility, that perhaps some people can actually be cured after having metastatic lung cancer, let. Me just say that, it's a small, percentage. Of people who have these kinds, of outstanding, responses. But. It's. Not, something new so. The. For, instance for frontline treatment if you. Have high, expression of the ligand, which, is, somewhat. Predictive. Of response. Not, exclusively. Predictive, of response then. The overall progression-free, survival is. Almost, doubled, but. Again, what's really exciting is that people. Who respond, tend to stay responding. For a long period of time, so. That's in a. Small. Nutshell, the. Landscape. For, treatment, so, as exciting. As it is. People. Don't. Really get cured and I will never walk into a patient's, room and, say well I'm gonna give you immunotherapy. And I think you have a chance at a cure, you. Know I'm, hoping that, that is going to turn out to be the case eventually but. Essentially. If you have stage 4 disease. That. Is a death sentence, so what can we do so, obviously we're going to continue to work, on the therapeutics, but I'm going to now turn to, prevention, and early, detection. So. Smoking. Important. To know obviously, it's the risk factor, but. The. Converse. Question, is so if you smoke you increase, your. Chances. Of lung cancer if you stop, smoking do you decrease your chances, it's, not the, answer is not as simple. As you would think so, these are the long-term results, of a study. I was actually study of COPD called, the lung Health Study 15-year, 14.5. Year follow-up, and what. You see here is the, death rate okay. 400,000. From. Lung cancer from, other diseases okay, if you, continue, to smoke it's, 3.5 for. Certain. In a hundred thousand four one thousand, if you. Quit. Okay. Then. It's about half. Of that and if. You. Intermittently. Quit, it, was somewhere in the middle so, if you've quit for 15 years, your, death rate from, lung cancer is really, decreased. If you, if I had shown you the, data from, the 5-year, follow-up there'd. Be no difference and the. Reason for this is because. Lung. Cancer. Tobacco. Damages. The epithelium, there's genetic damage. There's slow turnover, and so if you continue to smoke your risk continues, to go up if you stop smoking you live. With the risk that you had so if you continue to smoke there's your rate of lung cancer if you stop smoking it, toes, okay. So, should. You quit smoking, absolutely. The, sooner the, better but.
If You quit smoking today and you've been smoking for thirty years don't, think that your risk. Of lung cancer goes down quickly. It doesn't goes, down for cardiovascular disease, much. Faster than for lung cancer. Okay. So let's talk a little bit about prevention, and. Really. About what we call, because. You will continue to be at risk about, cancer chemoprevention. This. Was just defined. Many. Years ago by Michael, sporran who had been here at NCI at the time as. The use of natural, or synthetic agents. To suppress, or reverse the process of carcinogenesis. As I. Tried to tell you you get all this damage to the respiratory epithelium. And that, progresses, over time so. With chemo prevention you, try to undo. The damage so, lesions, that are partially, progressed, that are premia plastic, you want to regress those you. Want to prevent new lesions from, getting to the point that they're premia. Plastic, and. To. Suppress, those that are near plastic, already and so, the rationale, there for for trying to prevent lung cancer is because no matter what I told you and how exciting. Immunotherapy. And targeted, therapy, is the, lung cancer survival, still is awful, and unacceptable, okay. And. We know from other diseases, that, actually, cancer, is preventable so, I don't know that you've had the breast cancer lecture, yet and whether, you've, heard about the tamoxifen, and raloxifene trials. But, these are trials in women, who have who, were at risk but did not have breast, cancer yet, and you can decrease the risk of subsequent. Breast cancer by 50% so. And, of course we can model, this in animals, in many different ways so, it's, possible. Question. Is how you do it, knowing. That there is this long, preclinical. Phase with, these increasing. Histologic. And molecular abnormalities. Primarily. Due to tobacco exposure. Means. That there's an identifiable, population. You could study and hopefully, intervene. In. So. When. Do we want to intervene well, it, depends, or why it depends, on the efficacy if we can intervene, when you have metastatic disease that's. When you want to do it right those, are the people who need it but we, know that we do better with early stage cancer than, with late stage cancer, we can cure people by surgery, and adjuvant, chemotherapy. We. Don't cure them with metastatic, disease, so. We, presume. That the precursor. The pre new plastic, lesions, are, more. Curable, than the invasive, ones because they tend to be less molecularly. Complex. That's. A hypothesis. Okay. Can. We prevent the carcinogen, induced DNA damage in the first place, probably. But you'd need to start, that when you're born yeah, you're probably in utero and, we, need to be aware of the fact that I told, you about the various driver mutations, many of which are in, non-smoking. Ever smokers, but some of which occur in smokers. And there, are there different, pathways, to getting something that looks similar under the microscope so. You, need to have interventions that, can, address, these, multiple, pathways, of, carcinogenesis. So. That's one issue you, need efficacy. Which. We think earlier is better than later we, need to look at toxicity, because, if you're talking about people who are have, metastatic disease you.
Can Get away with, bone, marrow transplants. And you, know things, chemotherapy. Regimens, that have very high rates of. Toxicity. Including. Having. People. Wind up in the ICU not infrequently, that's. Not the. Case and people who feel, healthy who may never develop cancer, right, so the toxicity, has to, be acceptable. Both short-term and long-term, you, can't increase. The risk. Of other. Abnormalities. Like cardiovascular disease, okay. A. Third. Consideration. Is the size of the target population you, know there are 44, million current, smokers, and about as many former, smokers in the u.s. it's, not obviously. They're not going to develop. Lung, cancer in their lifetime, I want an eight never. Mind, right, now so. You have to be able to. Figure. Out who are the highest risk smokers. And, of course we cannot identify the, non-smokers. The never smokers who, are addressed because it's. A substantial, number of people but it's still a relatively rare population. So. These, are all issues and, cost is always an issue so. How do we identify agents. Well where, we know the mechanism and, I already told you there are many different mechanisms for. Lung cancer we. Can do better HPV, vaccine, and cervical cancer you, know that's, not. To. Belittle. How, difficult, it was to get to this point but you understand the pathogenesis you, have a vaccine you, know you could prevent. Essentially. All almost. All cervical, cancer so. With. Lung cancer that's, a lot more difficult so. We turn to preclinical, models, we. Look at epidemic G, and, I'll give you some examples of all of this and. We look at secondary. Endpoints, from clinical trials this, is how we found. Tamoxifen. And raloxifene for, breast cancer. Okay. So, target. Selection cohort. Selection, risk. Benefit. What. Are the endpoints that we look at and to. Design the clinical trials the right way those are the major issues that's, what I really. Spend, my, days, worrying. About, okay. So. I'll tell you a couple of stories of things that we've tried, to develop one. Of them and. Not just we at NCI, but others in the field and one, of them is the inflammation. Story, there's. A lot of data going back to the 70s, showing. A role for steroids, in cancer prevention this is primary, primarily. In animal, models. This. Moved, on to lung models, oral. And inhaled steroids, the work of the late, I would say greatly wattenberg who. Really was a mentor. The. Epidemiology. Has been not, as strong, but. Most. Steroid, use has, been in the setting of asthma which is not the high-risk condition, COPD. Is the high-risk conditions, and. There's some studies suggesting, that those. Smokers. With, COPD, who, use steroids have a lower risk of cancer and, those who, did not. This. Is an example of an animal model this.
Is A, vinyl. Carbamate, it's, a if, you give vinyl, carbonate, to mice. You. Get. About. 80% decrease, in the number of, tumors, and depending, on the. Amount. In the diet, this, is budesonide, which is one type of steroid and. Not. Only do you get fewer tumors, but. Instead, of having, carcinomas. You, really, revert them back to adenoma. So you prevent, the progression decreased. Numbers, not. As, advanced. Histologically. So. How. Do you bring this to, people. Well, in. The bronchial, epithelium. We, know the progression to, spray the cell carcinoma. It goes through, varying, rates. Of dysplasia, of. Abnormalities. Until, you get to carcinoma, in situ and, then, invasive, carcinoma, and. These. Lesions can be identified, on bronchoscopy, and, they, tend, to progress to, more, severe. Lesions, if you follow them over time. Not. Only shows progress, in fact the majority, don't. It's. Hard to know which ones will but. The ones that are mild. Like, the metaplasia. Really. Will regress, as much, of the most, of the time and only, infrequently, progress. To, carcinoma. In situ, whereas. The severe, dysplasias, will. Still, regress, about half the time but, a third of the time they, go on to progress and the carcinoma in situ tend. To progress in, almost half the patients, so, the more severe the lesion, the more like, if you go back to look that you will actually get, to a cancer, this. Is an, interesting study that showed that in, people. With low, and high grade bronchial. Lesions, about. 1/3 developed, invasive, cancer, however, only, about 40% of those were at the site of that abnormality, the, other 60% were someplace else either along the bronchial, epithelial or. Even. In the periphery, of the lung as AB no carcinomas, so. This tells you that these lesions are, both, precursors. Because some of them will go on to develop cancer but, they're also the risk markers, for an abnormal, field because. Of tobacco exposure. So. This, is what we have focused on are these lesions because. You can go back as, hard as it is you. Can go back with repeated. Bronchoscopy, looking. In biopsy. And then, asking, do, these lesions get better are there new lesions so. We took inhaled, budesonide, which is what the mouse study showed, was. The, data that I showed, 212. Smokers, who had dysplasia. In the, bronchial. Epithelium, they also underwent a, helical. CT. They. Were given budesonide, or placebo inhaled. For 6 months and then we went back we brung them and we repeated, CTS, and. To see how, how, well they did this, is work of Stephen lamb in British, Columbia. Cancer Agency who. Is one. Of the few who can do these types of studies. The. Results the, data from, that study are. That we, really did not have an effect on bronchial, dysplasia. Although. The. City detective lung nodules, actually. Tended, to resolve, more, frequently, in those. Who had. Budesonide. Versus, those who did not now. Why would, this be well the, animal, model, that, we used, which, was the really the only type, of model, available at the time, gave. Mice, little, adenoma. Not squamous, cell and. We were looking at in people in retrospect. Were the, bronchiole be tibias which gives rise to squamous. Cell carcinomas. Not. The ad knows what you find in the periphery, of the lung of the CT detective well no nodules. Are, more, likely to give rise to ad no carcinomas, so. We. Figure. This out. Retrospectively. And. Decided. To look, in the periphery, with the advent, of, low-dose. CT scans. And so. This, is the sort of the next study in this. Process. Where, we took people who were undergoing CT screening this. Is the work of Julia Verne a scene colleagues, in. Milan, at. The European, Institute of oncology, they, had a screening cohort, and we, randomized. People to get inhaled budesonide, or placebo for the year in between, their repeated, see CT, screens. Now. This is the first time we've ever actually looked, consecutively. In the peripheral, lung and we found many different types of nodules, and obviously. We ruled other people had actual. Lung cancer so we were only looking at the what's called the indeterminate. Nodules, which you would follow but not take, out because they're small, so. The solid nodules, you know that had been there stayed, there didn't, change in size the. Non-solid, nodules however. Did. Change in size they got smaller and as we've and, this was not statistically significant, because this was only about 30 percent of the people who, had these kinds of nodules, but, as we followed them over time they, got a year, of inhaled budesonide, and then, they continue to get screenings.
As. We followed them over time those, who had budesonide. Still, continued to get a little bit smaller while, those who had placebo. Did not, so. Not. A homerun but. At least telling us how. Maybe we can do these studies better so, what are these nodules that, I'm talking about they look like this there are these ground-glass, not. Fully solid nodules, under. The microscope some, of them look like this this is what's called a typical adenomatous, hyperplasia. Aah. It is. About. Quarter. To half, of these. Kinds, of nodules. Will. Be a HS. Others could. Be carcinomas. Or some fibrosis, or various other pathologies, and. It. Turns out that the. Belief is that these nodules some. Of them are actually precursors. So this is one study that followed. Patients. Over time with. These ground-glass. Opacities, and basically. About 1%, of them will, become invasive, over. Time, some. Of them will, become what's. Considered. Non. Invasive, but. Cancers. ADNA. Carcinoma, in situ. Minimally. Invasive, adenocarcinoma. So. These, nodules are. Some. Of them are precursors, I'll, give you sort of a real-life example and, it shows the complexity, this is actually, a, friend, of the family also, became, one of our patients. Woman. Who. Developed. Chest pain went, to the ER they. Thought that maybe she had a pulmonary embolus, she didn't they did a CT scan, and. They found this this, is a ground-glass, opacity. Okay, this is of. April. Of 2004. As, is. The. Standard. Of care she, was told to come back in three months didn't. Really change a whole lot a whole lot she, was told to come back in a year and you know life takes you in various ways so she didn't, then. Wound up in the ER again six, years later and now. Again. Chest. Pain didn't. Turn out to be anything CT. Scans showed this this is. Now a little bit bigger than, this so. She went back a, year later yet, again and now, it's more solid, and so, this turned out to be an invasive adenocarcinoma. With. Areas, of atypical. Adenomatous hyperplasia, so this, was clearly the precursor. Here's. The cancer. Seven years okay. So. If, we had done a study using. Her as one. Of the participants, we, would have gone from this to. This, you. Know I mean somewhere in between it. Was we will probably gone from this to something that looks just like it in a year so. That's part of the difficulty, of doing, the studies, so. We've gone back to ask the question a little bit more again if you, have non-solid, nodules what's. The risk of developing lung, cancer these, are data from the National lung, screening trial. Which was done, which. Was coordinated. From the National, Cancer Institute, and, the. Bottom line is that we looked at people who had these small nodules. Ground-glass. Versus. Other type, of. Abnormalities. Solid, abnormalities. And five. Or more, years out they had about a three-fold, increased risk of lung cancer, so some of these ground-glass, nodules. Are, lung. Cancer precursors, others. Are not so what are we doing now so now we're looking at aspirin, okay, so to continue, the, story. Of, inflammation. And, the, reason we've turned to aspirin, from, inhaled, drugs is because number one it gets every, place more evenly with a systemic, but. It's really from these studies by, Peter. Rothwell, and colleagues, and it's a whole series of studies, looking. At the long-term, outcomes. And people who were randomized, to receive, aspirin. Or placebo for cardiovascular disease. Many. Of whom by the way smoked these, were old studies, and when. You look at the long-term outcomes. In terms of cancer, deaths, five. Years or more out you, see that the curves diverge, and you, get about a 20% decrease. Risk of cancer, death five. Years or more out and, the longer, the, better and if, you look at various. Types, of cancer. This. Little teeny one here, is lung, cancer actually, this curve diverges. Within, about two years again, this is cancer death and this, is long adenocarcinoma, where, you have about a 30% decrease. In cancer, deaths by five years so. We, went back with, dr. Varanasi and colleagues, and in Milan, to. Ask if, we take people now with these pre, malignant. Lesions. The, AHS, though excuse. Me. The. Ground glass capacities. So, not the solid nodules, we. Screened them because, they're part of a screening program we, give them aspirin, for a year, can, we get.
A Better outcome than with inhaled, budesonide. And this is still ongoing and we. Also decided, to look a little bit more. Molecularly. To. See what, is the effect of aspirin, short-term and so in, this study what we're doing is we're. Doing nasal. Swabs so there's a whole series. Of data showing, that there. Is there abnormalities. That you can detect by. Gene expression. Various. Signatures, that. Are associated with smoking versus, non smoking those, who have cancer versus, some where else, in the, lung. Versus, those who don't and, that, these signatures. Are similar, in the. Nasal epithelium as, they are in the bronchial epithelium, so, we're looking at the short-term, exposures. To aspirin, to, complement, the. CT nodule, study. I'm. Going to tell you one other very short story, because. Again it's not a drug that we're ready to tell. People you can use but. Because it shows, how we're, changing how. We do these studies so. This is my inositol, which is actually, a glucose, isomer, source, of several signaling. Molecules. It's, actually found, in and rice and, baby's. Milk and various. Grains and so on, it's. It's a food constituent. It's. Actually. On the, list of what the US. FDA calls, grass. Generally. Regarded, as safe which. I think for prevention is very important, and. So, there been many studies again, the work of wattenberg and, Steve Hecht that. Showed that. Myo-inositol. In, animals, can. Inhibit. Carcinogen. Induce tumors. Both. In smoking. Exposed, animals. As well as in carcinogen. Animals. If. You. Combine. It with steroids. You really, increase the efficacy so. We decided to go with our colleague, Steven Lam in Canada. To, do a phase one study figure out what can we do, in people, and. Found, a tolerable dose of 18 grams per day this. Was an, uncontrolled. Study, because. We wanted, to know the, dose, that is correct, but, people underwent bronchoscopy and compared. To historical control. Which, is a dangerous thing to do you. Can do it as. To. Push. You in the right direction but.
You Can't, assume. That this is the correct answer, so, compared to the historical controls. We, found a very very, high rate of. Regression. Of bronchial, dysplasia. We, found the dose. Preliminary. Data. We. Were lucky enough to. Have. Bronchial. Brushings, from. These Airways and. Ave. Sparrow and Boston, University looked. At gene. Expression in, these bronchial, Airways and, showed. Through, a very, long series of elegant, studies that. The pi3, kinase, pathway, is activated. This is now in the normal bronchial, epithelium, in smokers, with, dysplasia, in. In. To a much greater degree, than, healthy. Smokers, or non-smokers. And. Myo-inositol. There's. A small. Number of patients, inhibited. This pi3, kinase activation in, this, normal, bronchial, epithelium, so. This, opens. Up the possibility two. Possibilities. One how, important is the pi3 kinase, well actually pi3, kinase is frequently, activated. In squamous, cell carcinomas. Head neck long, as well. So. Does that. Identify. The, truly, high-risk, smokers, not, sure yet, but, it also gives us this new clinical, trials model where we can do these shorter studies and look, at different, outcomes, not the bronchial, dysplasia, but actually. Looking, and gene expression signatures. So. That's how we're trying to move forward so to. Look at that we did. The face to be study which means that we randomized, people to my inositol, Zeebo, everybody. Got a bronchoscopy. Pre. And post-treatment, and also. Gene, expression, signature was assessed from the. Normal. Bronchial, brushings. Primary. Endpoint was. Not nearly, as. Promising. As, was. The. Small. Phase one study which is why I cautioned, you not to, believe. That so, there was a. Slightly. Higher, rate. Of complete. Regression. With, myo-inositol, but, also of progression. With, myo-inositol, in all, subjects. So. It, really came out a, wash. So. This was really a negative phase 2b study. With. Increasing. Abnormalities. There's, increasing. Ki67. So. Proliferation. Placebo. Versus, myo-inositol. Not. Statistically, significant, but definitely a trend or it's more inhibition. Of proliferation with. Myo-inositol and. Downstream. Of pi3, kinase Akt pathway, the. Activation. Was decreased, in the, complete responders. And not not. On the others. Similar. Data to the phase one, study but. Doesn't. Help us figure out who's, going to respond, and why so. We'll continue to. Look. At all this wealth of information to, try to figure out whether we can identify who. Should get my own acetal, it's. Obviously not for all, smokers. Very, heterogeneous response. And. We need to do better, all. Right so. That's those, are examples, of how we are doing studies, there, is no approved. Strategy. For, lung, cancer prevention. Right, now other. Than stop, smoking, and that'll, help you years down the line how. About early detection so. Very quickly. Nothing. In this life is free okay. And so the issues with lung cancer screening are. Also. Complex, and, that's, because, you're looking at, various. Time, points and. Our. Screening. Is subject, to various, biases. Something. Called lead. Time bias where. Because you're screaming you're. Finding. The, diagnosis, at an earlier time but, you're not actually postponing. The. Time, of death survival. Only appears, longer, there's. Something called length bias you're. More likely to find the indolent, disease as. Opposed to which. Has a longer preclinical. Phase, it's, well known that with. Mammography, that. The people who have really, bad disease, often, pop up in between the mammograms. Okay. There's. Something called over diagnosis, you identify. Actually. Clinically unimportant, lesions they look like answer, but they would have never been diagnosed, and they would never would have caused the person's deaths and, of course anytime you, do anything to people there's, morbidity.
There's Mortality. And. And. There's a cost. So. This. Is the PLCO, chest, x-ray randomized. Trial ok can doing, chest x-rays reduce. Lung. Cancer mortality one, hundred fifty four thousand plus participants. Just. A one chest x-ray vs. usual, care. For, screens thirteen, year follow-up no, change to mortality, okay. So just, getting a chest x-ray isn't going to help you how. About CT, so, CT, was developed in the 90s and. So. We have the National, lung screening trial. The N LST which, is really, the biggest. Random. Control, trial of CT, screening. 53,000. Smokers. Current, and former at least 30 pack years have, to have quit within, 15 years, and. That and this was a, randomization. Between. Helical, CT and chest, x-ray and so even before we knew the chest x-ray was definitely, not helpful. Okay. The, results, were, that there were about a quarter, of people had a positive test okay. 350. For lung cancers chest, x-ray many, fewer positive. Tests, 442. Lung cancer deaths overall. A 20%. Reduction in, lung cancer mortality highly. Significant, there was even a significant, reduction in all cause mortality. And. This is just a picture showing. Cumulative. Lung cancer cases there's. CT. Versus, chest x-ray but, if you're looking at deaths it's the. Chest x-ray that's on top that's what you want to see you detect more and you. Have fewer deaths so. Good, so. This. Is now being. Worked. On to, make it. Person. Friendly, and to. Optimize, because. That's, screening, a lot of people to get a 20%, decrease in mortality so. This is example, of one study that looked, at various. Parameters. Age sex, family, history, and Aseema types. Of nodules, and could, tell you the, risk of having a lung cancer, based, on that first screening, CT, this. Is work from Canada again so, this would allow us to identify those at highest risk for, subsequent. Screenings, as well. As potentially, for future, phase three chemo prevention trials. So, what we want to do is we want to identify, those. Who are most likely to actually have, lung. Cancer, subsequently. And. Eventually. Dot those, are the people we want to intervene it okay.
At Just, again. Screening. Should be a whole different. Lecture. So let me just sum. Up then, there's. Been huge, progress. Since. Since. The time I entered and, came. To NCI, which. You, didn't mention the year Terry, in. Understanding. Lung cancer the. Genesis, of lung cancer in a molecular way. Precision. Medicine is applicable, now, to a significant. But still small, subset. Of advanced. Age among cancer patients, there's clearly, increased. Survival, in this, subgroup, we're. Now in the early days of immunotherapy but, already there's, been very. Significant. Prolongation. Of survival, again, in a small subset, of patients and, we, can decrease lung cancer mortality with. Early, detection with. Helical CT so. Work. Is ongoing, and, it's. It's. Actually a. There's. An enthusiasm, that. Was not there 20 years ago, but. There's still a lot of work to be done and, with. That I. Will. Go back to my prevention, routes ounce. Of, prevention is worth a pound of cure and just. To thank a number of people who've, actually done the work and, I'm, happy to answer questions. Yes. Sir. Its. Oral it's, a powder. Right. Good. Question. We have no idea okay and, so, that's the kind of study that you. Would want to do. Well. You would want to do it in two settings one is that if you really think that there is a sub population, who are truly, responding. And you want to understand, the mechanisms, or. If. You, think, that there's a problem there that you can fix to. Help. The efficacy, then. You would want to do the studies until. We get a strong. Enough signal. That. This, really is a good. Chemo. Preventative agent I'm not sure it's worth looking. Into it but, it's a very good question as to whether that's what's going on. No. Not. For, lung, cancer prevention. You know there are guidelines for. Using. Aspirin for cardiovascular disease and, for those who are at risk for cardiovascular disease, which smokers. Are increased. Risk but, you know with to, a specified. Amount to. Actually, use. Aspirin, for colon cancer prevention but. There's and that's actually in, the guidelines now but it's you know it's for ages, 50, to 59. Or something and consider it you know in the next age group. There's. Really not enough data and the. History. Of cancer. Prevention is, littered, with good. Intentions. Of studies. You know the beta carotene studies and I didn't talk about where. The outcome, was worse but, the intervention, as opposed to better so, until, you really have good clinical, data I would not do that unless she's, at a high cardiovascular. Risk. But. Tell her to stop smoking. All. Right. You. Okay. All. Right so our next speakers here and, we'll get started. So led knickers he's. A senior, senior investigator, in the urologic, oncology branch. He. Got his PhD from the University, of Connecticut, did. Postdoctoral, training, at NIH, and joined the NCI in. 1981. He's chief of the tumor, cell biology, section and. He's. Going to talk to us about. Targeting. Molecular, chaperones, in cancer. Lessons. Learned from hsp90. Thank. You teri. It's. A pleasure to talk to you you guys about, this this is a little bit of a. Change of topic from the last speaker. But. I think. It, provides. Some interesting. Examples. Of how novel, targets. For cancer treatment. May. It first appeared, to be surprising. Tarp. Targets. And then perhaps. The expectation. Is to have great results, and when the. Great clinical. Data don't, come, come, in one, needs to start looking, for explanations and. Usually. The explanation. Is that things are more complicated, than, we thought and that's what I hope, to show you here. But. It. Shouldn't stop us from continuing to explore, to, understand.
What The deficiencies. Are. Some. Of the things we know now we didn't, know 20. Years ago when, we started developing these, agents, and, and. I, think. That, the future suggests. That. Chaperone. Inhibitors, may still be useful both. For cancer. And for other diseases, if, used, appropriately, so, with. That in mind I'm. Going to talk to you about a, particular, chaperone. That I've been working on since the early 1990s. Hsp90. Although. We didn't so much appreciate, it then hsp90. And other molecular chaperones. Are just part of. A. Group of. Effector. Proteins that. Make up the. Pretty oh stasis. Network, or pn and, this. Network which, includes the proteosome, tov EG. Directed. Proteins. The unfolded, protein response in. The endoplasmic reticulum. My. Toffee g etc. Are. All. Means. That, the cell and the and the organism. Uses, to. Cope with environmental. Stress. Which. Leads, to, damage. Of proteins, in the cell this has to be dealt with if it's not dealt with. This. Accumulation. Of, miss folded, proteins. Is going to kill the cell. So. Look at looked at from that perspective i suppose it's not surprising, that. Cancer, cells which as I'll show you suffer, from all sorts, of, environmental. Stress. Pretty. Much all the time, have. Grown very dependent. Not only on hsp90. But on the pretty o stasis, network, in general and. There. Are. Very. Few but some examples of, drugs that target this network, hsp90. Inhibitors have, been in the clinic, since the late 1990s. A. Number, of proteasome, inhibitors. Have been in the clinic for almost as long and, in fact. Two. Of these have been approved by the fda for certain, types of cancers, so. Understanding. That the proto stasis, network, is the target, is, is. Appreciating. The fact that the infrastructure. Which. Allows cancer, cells to survive and, multiply. May. In fact be, a useful target. For treatment. So. Normal cells as I just mentioned, hsp90. In. Conjunction, with other members, of the PN helps, to maintain protein. Homeostasis or. Protea stasis, in. Cancer. Cells this, becomes. A much more complicated, and, more difficult, task for, both. Hsp90. And the produ stasis, network in general, because. There, are many, mutated. Overexpressed. Or. Activated. Uncle, proteins, in these cells, that are inherently, unstable don't. Fold, easily and, need. To either be. Chaperone. Through multiple, rounds of chaperoning. To, allow. Them to fold or if, the cell senses that they're never going to fold then to target, them for, degradation. In. The proteome, in particular so. That they don't accumulate. And and cause toxicity. So. In, terms of of hsp90. It's been known for a long time these, are data taken, from a paper, in 2013. But, yeast. Experiments. Going back to the early 1990s. Confirmed. That yeast, that are growing at optimal. Temperature. And growth conditions. Have. Maybe 10 fold excess the amount of hsp90. That they need in order for. Survival. And for growth and that's, just shown, here. In, in this graph, which. Looks at expression, level of hsp90. On the x axis and relative. Growth rate on the y axis and, you can see that relative. Growth rate in in these yeast is not impacted. Until. Levels. Of hsp90. Are reduced, by, upwards. Of 90%. So. These, are under optimal. Conditions. If. You, look at this hsp90. Point, mutation. In the red box. This. Mutation. Weakens. The activity, of hsp90. It doesn't destroy, it and as. You see here in this yeast dilution. Assay, where. Yeast. Cultures, are spotted, at a. Number. Of serial, dilutions. Going. From left to right, and then one observes, their growth, just. By looking at how dense, these white circles, are and, 30. Degrees, which is the optimal, temperature, for East. This. This, function. Compromising. Mutation, has no impact, in ease growth but. If you switch the yeast to 16. Degrees. Which. Is definitely not optimal. For their growth, while. They're. Not affected, if they express wild-type ages, be 90 or these other two mutants, not in the red box, but. The, mutant in the red box is now no longer able to. Support. The growth, and viability, of these yeast. Another. Way of looking at this is by expression, strength, now you can do a similar.
Experiment. To. Comparing. The 30 degrees, to 16, degrees you can do it all at 30 degrees, again optimal, temperature, but. You can vary the expression, of hsp90. Like, I showed in the original. Slide. Panel, on the left of the first slide. So. You, can see if you look at the wild-type, which. Is in the green box, up at the top. Pretty. Much you can reduce. Hsp90. Expression. To. Well. Less than 10% of, normal, and the. Yellow cholera suggests, shows that the yeast are able to tolerate that in a growing fine. But. If you look at the W. 585, T mutation. Which is shown. And the. Red box you. Can see that at 30. Degrees at optimal. Concentration. Like I showed you in the previous slide, the yeast are happy, but, as you begin to decrease expression. Even though growth conditions, are optimal, it begins. To not work and to not, support East, growth so. The. Reason. I'm showing you all this is is that the bottom line is, that in East as well as in human cells there's. Generally, an excess, a large, excess. Of. Hsp90. You. Can reduce it a lot and have no obvious, effects. But. If. You. Stress, the yeast either by giving, them an HSB, 90 that is not completely. Active. Or. If, you, put. Them at a temperature that they don't like they now become very dependent, on, optimal. Expression. And. One. Can imagine a similar situation for. Cancer, cells so. The, Green Line is. Showing. What. Would happen to a normal cell if you reduce the hsp90. You. Can you you'll get to a point where, it's going to have an effect because hsp90. Is essential. For growth, but. Normal. Cells don't need all the hsp90. That they have in normal cells and tissues hsp90. Makes, up, somewhere. Around 3 percent of, the total protein, of the cell so it's quite a lot it's in micro molar. Concentration. In cells and they. Can get by with a lot less. But. If you stress the cell or instead. If you look at a cancer, cell which, as. I'll show you in a minute is essentially. Stressed, all the time. Then, they can tolerate only a little reduction, in hsp90. Levels, before, you see significant. Effects on growth. And viability, or. Fitness, as as shown here on the y-axis. Now. It's. Not. Exactly. Correct. To, equate. Expression. With, inhibition. But. An analogy, can be made. Suggesting. That you can inhibit, hsp90. To, a sufficient. Degree where, you're going to see in effect, in cancerous cells while. You see minimal, impact, on normal, cells and, over.
The Years and in published. Papers this is in fact what we see so. Cancer. Cells in general tend, to be more sensitive. To, pharmacologic. And a vision of hsp90. Compared, to normal cells and. A. Likely. Reason for this is that hsp90. Is, one, of the main response. Mechanisms. That the cell and. The organism, has two, environmental. Stresses, some, of which that are common, to cancer, are shown here, on the. Left-hand side. Cancers. Usually outgrow, their blood supply and so nutrients, stress is, a common, factor. There's, proteotoxic, stress, because. There's. Usually, a lot of reactive, oxygen in. Cancer, cells they're. Hypoxic. So they're, not getting, enough oxygen. To, have, the mitochondria. Function. And, optimal. Activity. And many. If not most, cancers. Are. Aneuploid. And are characterized. By extreme, genetic. Instability. This. Leads, in turn, to. The synthesis, of. Mutated. Proteins. That are not able to fold and. Must be dealt with by the cell, either to. Chaperone. Them until they're folded, or to. Target, them for degradation, and, hsp90. Is involved, in both of those processes, so. All. Of these stresses. Impinge, on hsp90. And hsp90. In turn. Controls. The fate of a host of Cline proteins, that are signaling, proteins. For. Example the, one shown on the right hand part, of the slide that. Mediate. A lot of the processes, important, for cancer cell growth survival. Metastasis. Etc. The. Gray. Boxes. Describe. What. Are now accepted. As the, hallmarks. Of a cancer cell that distinguish. Cancer. From normal, and you. Can see that all of these of all. The hallmarks, that are shown here there. Are client, proteins, that depend on hsp90. That. Are, important. For each. Of them so, that you would think that by targeting, hsp90. You, can have, a major impact on, the viability of cancer. Cells in vitro and, in, vivo and. A, correlate. Of this would be to to suppose, that if. You. Stage. Cancers. By the amount of hsp90. They have the more hsp90. Expressed. In a cancer. The. More aggressive the cancer might, be and and, the worse the, survival. Or the prognosis, of the patient, would be and this, in fact has been shown, by. Looking at tumor tissue in. That. That's taken from patients, for a number of different kinds of cancers. Including. Non small cell lung cancer. Which I'm not going to show you today but also in breast cancer. Which. I'm showing, you on this slide. Where. If, you, stratify, the. Tumor, samples, from patients. Which. With triple negative breast. Cancer. To, and. Make a cut-off we're above, that is arbitrarily, called, high, hsp90. And below that as low hsp90. You. Can see that in. Tumors. Where. Hsp90. Is, upregulated. Significantly. Compared. To the, remainder. Of the population. Per. Sensor survival. Is markedly. Reduced. In. These patients, so. Expression. Level of hsp90. Is a negative, prognostic. And an indicator. Of cancer. Aggressiveness. And. Survival. It's a prognostic. Indicator, of aggressiveness. It's a negative prognostic. Indicator, of survived. So. Our. Idea was that inhibiting. Hsp90. Like, knocking, down expression. Of hsp90. In simple, systems like yeast should. Compromise. Cancer. Cell robustness. Robustness. And this. In fact turns, out to be the case, this. Is an experiment, we. Published. A long time ago now, that. Shows, that this, prototypical. Hsp90. Inhibitors 1780, which is the first inhuman, hsp90. Inhibitor, is very. Effective, as a radio, sensitizing. Agent. We're. Looking. Here at at, a, couple. Of lines HeLa, cells, and, see. How cells and, you can see the, doses, of radiation on. The x-axis and the surviving, fraction, on the. Y axis and the y axis make. Note is in a, log scale. So. You, can, see that at this dose of radiation. That. Hit. The HeLa cells and the sea hares hot, cells which are both lines, derive from from. Cervical cancer. Are. Sensitive. But, certainly. There's, a significant. Fraction of, cells that remain, after. Irradiation, if, you. Add in, low. Doses, these are nano molar concentrations.
Of This, hsp90. Inhibitor. You, can see that you markedly, improve. The. Toxicity. Of a radiation and this definitely, is combinatorial. Because. In the, absence, of radiation. This dose of hsp90. Inhibitor, isn't, doing anything by itself. This. Is this effect is is time-dependent. If you look at the panel on the right you, can see that the. Longer the pre, exposure, to. Hsp90. Inhibitor, the, better the sensitizing. Effect, going from four, hours. Pre, exposure which, essentially, has no sense sensitizing. Effect to, 12 hours pre exposure which has a a significant. Effect, you. Get logs more, cell kill. So. So. Far the, data. Suggest. And. Additional. Information, that, has come out since this support, the fact that in. Indeed, you, can affect the robustness. Of cancer, cells with. Relatively. Low dose hsp90. Inhibitors. So. How do hsp90. Inhibitors, inhibit. Hsp90. So, it. Was unclear when, we, got into this, and we identified, the first small molecule, inhibitor, of. Hsp90. Which is a version, of, 17, AG, in. 1994. It. Was unclear whether hsp90. Was, a chaperone, that required, ATP. Or or not, there are examples, of both kinds of chaperones. Using. These small molecule, inhibitors, it's. Become, very clear, that, ATP, binding and hydrolysis. Are essential, for hsp90. Function. And I'll show you why. As. It. As it happens. These. First, inhibitors. Of hsp90. That were identified. Seven. Teenage years the derivative, of delta myosin, and then. A second, structurally. Distinct. Inhibitor. Is called reduce, akahl and those are both shown on the right this, is the N domain, ATP, binding, pocket, of hsp90. And, you can see ATP, bound here. On the, left. And. To, everybody, surprised, these small molecule, inhibitors. Bind, in the ATP, binding, pocket. So. One. Reason why it. Was controversial, up until this point whether. Hsp90. Bound. ATP, required, ATP. Etc. Is that. Its, binding, affinity. For ATP is extremely. Poor and its. ATP, hydrolyzes, activity. Is extremely, poor but. With these drugs we can now specifically, measure. Hsp90. Dependent. ATPase. Activity because. Only. The. ATP. Bound to hsp90. Is going to be affected. So, contaminating. Proteins, and preparations. Like kinase, etc, with much higher ATP. A's, activity. Are. Not going to be inhibited, by by, these drugs and you can see the very small amount of, ATPase.
Activity Coming, from hsp90. Is fully inhibited, by these agents. So. Hsp90. Exists, as a dimer the. Dimerization, domain is, in the c terminal, part of the protein. ATP. Binds to the N terminal, part shown in blue and. In. The. Presence. Of ATP hsp90. Undergoes, a series, of conformational. Changes, sorry. Which. Is important, for ATP, hydrolysis. And for chaperoning, of clients, in. The. Presence, of these hsp90. Inhibitors, however. ATP. Can't bind because the inhibitor, is a much higher affinity, for this binding site then does the nucleotide, and. Hsp90. Remains, in this inactive. Conformation. Which. Leads to the. Degradation of, any client, proteins, bound to hsp90. Via. The proteasome and we, had identify. To e3, light, ligases. That, ubiquitinated. Proteins. So. That they are recognized, and degraded, by the proteasome. Both. Chip, and : five actually. Associate. With hsp90. And and. Are responsible, for ubiquitin. 18 clients. That, are not allowed, to proceed. Through, a chaperone, cycle. Because. Hsp90. Inhibitors are, blocking it. So. Development. Of hsp90, inhibitors picked, up quite, dramatically. After. We. Reported, the, first one in 1994. And. You. Can just see here over the. The. Last 25. Years or, so how. Many ages p90 inhibitors, have been developed, and all. Of the ones shown here have been evaluated in, clinical, trials. The. Little. Red. Top to, the. Panel. On the right hand side, is. A special, case of an, hsp90. Inhibitors that I'll get to in the last part of the talk but. Suffice it to say that a lot of hsp90. Inhibitors, have. Gone into clinical, trial and yet, no inhibitor. Has yet, been. Approved, by the FDA as. Having. Any single, agent, activity. And. These. Unfortunately. Are the few hsp90. Inhibitors that remain, in clinical. Trial as of August of this year so. Nowhere, near as many as. We're, shown on the previous slide, and, a couple of these trials, are simply, trials. For. Imaging. Of. Hsp90. With. Iota. Native versions, of these. Of, an hsp90. Inhibitor, but, there still remain a few, phase. 1 and phase 2 trials. That are actively recruiting. These. Combination. Trials. Are which, most of these on this table are, are. In combination. With previously. Approved. Drugs. That. Target this particular kind. Of, cancer. The indication, is shown on the right of the slide and so. You can see that now. Most. Of the clinical trials are not so much focusing, on hsp90. As a single, agent which, we would have. Suggested. From the beginning was, not the way to develop this drug it's best used in combination. Because what. The radiation experiment. Showed a long time ago is that we can affect the robustness, of a cell. Exposed. To something else when we put in an Aegis b9t, inhibitor, so. A, lot, of the agents, used in combination. Are kindnesses. But, not all of them are these. Trials, are open. And it's, it's hopeful. That. We'll see some interesting, results these, are primarily, phase, twos, there, are some phase ones also included. Here. So. About. Six, years ago interest. Was increased. That perhaps. We. Could develop an hsp90. Inhibitor. For. Clinical, use and. In, part based on this study. In her2, positive breast. Cancers. Where. Patients. Who had been treated with the antibody, to her to Herceptin. Initially. Respond, and and, then stop responding, and so everybody, was progressing. On the antibody, alone, now, her2, turns out to be a kinase. And it's very sensitive, to, hsp90.
Inhibitors Very, dependent, on hsp90. And when. These. People added. In 1780. AG. To Herceptin. Which remember, was no longer working, in these patients, there, was a, 63%. Increase. In. In, benefit. And a. Twenty six percent response. Rate which. Is actually, quite high so. People, got all excited that. Combination. With. A her2. Went. With it with an antibody that targets her2. May be a great use for ages, b19, and, this is still under investigation. Even, now, with, newer. More, synthetic, hsp90. Inhibitors. And. With different. Antibodies. To her, to. Some. Other interesting. Data. That. Is. Similar. Comes. From a phase 2 study in non-small-cell, lung, cancer. With. Gannett s PIP which is a more. Recent. Hsp90. Inhibitor, in. Patients. That are positive. For. Alcázar. Kinase, that is frequently, mutated. In. A. Reasonable. Percentage, of, lung, cancer, patients, and. All. Those green bars, you, can see, that. Are going down in, the, presence of Gannett, asthma as a single, agent here. Shows. That patients. Whose. Lung cancer, expresses. Out, mutation. Seems to be particularly. Responsive. To this hsp90. Inhibitor. The. Yellow bars are lung cancers, with wrasse mutations. A significant, number of them shows. Some response, as well. So. This, is also something that's ongoing. Some. Anecdotal, evidence of, single agent, activity of, can attest bourbon, in out positive. Lung. Cancer is shown here. Which. One of these is the light. So. This. Is circling, the tumor. Before. Treatment. With the hsp90, inhibitors you, see that this patient. First. Of all has stage 4 lung, cancer which, is very advanced. Received. Croissant. Nib which is an alkyne hibbott ER for one year and progressed, on that, and then, enrolled, in this trial looking at Kennett test bib and you can see that these tumors would. For no longer responsive, to an alkene hibbott ER nonetheless. Or, shrinking, after, three doses of, the hsp90. Inhibitor. So. There are trials underway right now. Combining. Alkyne, hitters, with hsp90. Inhibitors as, opposed, to just looking at. Hsp90. As a target, after, the patient, stops responding. To the alloc inhibitor. Here's. Another example in. A, lung, cancer, where the b-raf mutation b-raf. Is also a client of hsp90. This. Patient. Received. A lot of chemotherapy as, well as as targeted, agents. Including. A produce um inhibitor, and and. At this point was not responding, to anything but. After four cycles, of hsp90. Inhibitor, this lesion, went, from this size to, this size and. The. Duration was for more than a year so. There. Are definitely hints, of activity, even, single agent activity but. We're, asking these, hsp90. Inhibitors to now. Cause. Regression. Of tumors, that have stopped. Responding, to everything, else that's been tried and after they, develop. Resistance to each of these treatments, they get more and more aggressive. And this is a very high bar, for any, agent. To cross including. An hsp90. Inhibitor. This. Is a triple, negative breast cancer. Again. Receiving. A lot of treatment, and showing. What. Happens, after. Three. And a half months, after. After, four months of gannets husband, so. There's. A lot of anecdotal data, suggesting. That hsp90.
Inhibitors Should. Work in cancer. Why hasn't. Any haven't. Any been approved, yet so. We started looking at other things that. Hsp90. Inhibitors might, do that, that might be, counteractive. Of their anti-tumor, activity, and. One. Of the things that, that we and others have found is that hsp90. Inhibitors seem, to potentiate, the heat shock, response, by. Activating. The master, transcription. Factor, of the heat shock response ages. Of one. Which. Is activated, by the same kinds of stresses, that. 8h. Is p9d. Looks. For and and. But that's because hsp90. Itself, is a transcriptional. Target, of HS, f1 as is. Another molecular, chaperone, hsp70. So. What. We're showing here in a western blot in a cell line is that in increasing. Whoops. Increasing. Concentrations. Of gelt Adam Ison, has. Caused, the degradation of, Irby - also, known as her - which is that kinase that's very sensitive, but. At the same time that caused an increase in hsp70. Which is indicative of, this. Induction, of the heat, shock response mediated. By HS f1, and you'll, notice it's only the heat inducible. Hsp70. In the red box at the bottom, not, the constitutively. Expressed HSC. 70, whose, levels, are going up. Suggesting. This really is a transcriptional. Effect that's mediated, by HS, f1. And. If you look in patients, receiving in hsp90. Inhibitor, you. Can look at hsp70. Mrna induction. In. Lymphocytes. Of patients. And you. Can see from the the data shown here that there is a correlation, that's, quite strong between. The, dose of Aeg
2017-11-25 14:13


