Part 9 –New Technologies for Chemical Exposure Assessment
- Good afternoon, and welcome to the ninth Webinar in the Human Health Exposure Analysis Resource Programs Exposomics Webinar Series. My name is Jennifer Collins and I'm a health specialist in the Exposure Response and Technology Branch at the National Institute of Environmental Health Sciences, and the program officer for the HHEAR Coordinating Center. This webinar series covers various topics of importance to exposomics.
Today's webinar is focused on new technologies for chemical exposure assessments and will feature presentations from Dr. Manish Arora and Dr. Steven O'Connell on new methods for capturing chemical exposures using teeth, hair and wristbands and their applications in human exposome monitoring. Each presentation will be followed by an opportunity for questions focused on that presentation. And then at the end of the webinar, we will open it up to questions for either or both speakers. As you think of questions, feel free to type them in the Q&A box and I will read them to the speaker following their presentation. Alternatively, you may raise your hand and we will call on you to unmute and ask your question aloud.
Our first speaker today is Dr. Manish Arora. Dr. Arora is an environmental epidemiologist and exposure biologist, and the Edith J Baerwald professor and vice chairman of the Department of Environmental Medicine and Public Health at the Icahn School of Medicine at Mount Sinai. A founding member of the Mount Sinai Institute for Exposomic Research, Dr. Arora serves as director of its environmental exposure and Precision Environmental Medicine Laboratories. Leading a team of over 50 scientists who are advancing research in a vast array of diseases that are national health priorities, including autism, Lou Gehrig's, cancers, and the gastrointestinal disorders.
Dr. Arora, the virtual podium is all yours. - Thank you so much. I'll start by sharing my slides, and if you could confirm that you can see my slides in presentation mode please. Great. Alright. Thank you to the organizers for this opportunity.
It's a pleasure to be here. I'll start off by talking about the importance of the time dimension in environmental health research. I'll mainly focus on two technologies, teeth and hair biomarkers because I have direct experience with them.
But the following speaker will talk about another technology that also captures exposure over time. Before we do that, I just want to share some conflicts of interest. Some of the technologies I will present today have been commercialized by home institution, that is Mount Sinai. I want to take a bird's eye view of environmental health research and how we fit in in an era of precision medicine that has been largely dominated by genomics.
And this is one of my favorite studies. I've redrawn these graphs of a study done by Steve Rappoport many years ago that used genetics or twins and heritability to contrast the environmental versus a genetic contribution to disease. And as you can see in this graph, most of the diseases here have a big non-genetic or environmental component. But you don't have to focus just on diseases.
You know, increasingly, many of the studies I'm involved in, we are just looking at health trajectories. And again, some of the outcomes that I'm interested in also have major environmental contributors and cannot be studied solely from genetics. So sure, the environment is important. But there is one key difference between using a genetic or a genomics approach versus an exposomic approach.
And that is that time plays a much bigger role for us. Your base genetic sequence is set at conception, and sure there are some changes through epigenetics, but for the most part, you can analyze a person's genome at almost any time and get an answer. For us, exposures change over time, but also important to consider is that our response to exposure changes at different ages. In this diagram, the chemical description of a molecule above the individuals is that of cotinine or a biomarker of cigarette smoke.
If you're exposed as a fetus on the left or as an adult, your response to that exposure is going to be very different because your physiology has changed. And so the timing of when our environmental inputs are received and how we respond to them become very important. And for those of you who in the audience who are not from the exposomic field, I shared this one thing, which is very heartening to me that even in the general media, this idea that time is important, especially the early life, the fetal development and pregnancy and exposure that this time will shape our health for the rest of our life is very heartening that this is being recognized. However, as soon as we start considering the time dimension, we have to realize that this is a big challenge and it's a challenge that is often not fully addressed in the way we design studies.
My own interest is in looking at fetal exposure and how this affects children's brain development and then development or neurodevelopment in adulthood. So first of all, there's a challenge of how do we actually measure fetal exposure? How do we, you know, directly sample something that reflects fetal exposure, and not just rely on maternal biomarkers? But there's also an epidemiologic design issue here. If we wanted to do this with the best possible study design, and if we were studying conditions that are not that common, I'm not talking about rare conditions, but conditions that are single digit percentage prevalence, and autism is a condition that I do study, is very much like that. Then very soon we start looking at cohort studies that are in the thousands, sometime in the tens of thousands to just get enough sample size and power to study that disease.
Now we have many very, we have many other epidemiologic designs at our disposal, for example, enriched risk cohorts. Sure, so we can address some of that problem, but even if we did a much simpler study that requires a far smaller sample size, for example, a case control study, we overcome the issue of needing large sample sizes, but we still have this problem, how do we go back in time, many years back in time and study exposure that happened very early in life. You could ask yourself this question, what was my exposure before I was born? And not just based on a questionnaire answer, a true omic molecular answer to that question.
And it's something that I have grappled with for many years. And today I'll be presenting a technology that offers at least a partial solution to that. The answer to this, at least for me, arose from looking at something very much like this. If you look at a tree that's been cut down and you see all these growth rings, I'm sure everyone's seen this, and you start counting backwards. So if, let's say you count to about seven and you see that the ring is somehow disorganized, it's very thin or it's garbled, you know something happened seven years ago. Maybe that tree did not grow as well because there was a drought or there was some other exposure.
Part of my clinical training was in dentistry. So as soon as I had this realization, I could link it with one very simple fact that all baby teeth, in fact, all adult teeth also have growth rings in them. But one special quality of baby teeth is that they start forming in prenatal life.
They start mineralizing at the beginning of the second trimester. So every time a child sheds one of those 20 teeth that almost every child is going to shed, they're actually handing you fetal tissue. I know of no other example where someone in their childhood, up to the age of 12 and 13, is handing you a non-invasively collected sample of fetal tissue. So this certainly offers us many opportunities. I won't go into all the various laboratory methods we have developed using lasers and other nuclear beam methods, but I'll start off by giving you examples from sort of different domains of chemistry and what all we can measure and one example of how we've addressed an issue in autism spectrum disorder, a condition that I'm very interested in. So this is an example I've shared very often.
This is from a child who lives in Mexico and they gave us a tooth when they were about 10 years of age. The y-axis, we have the intensity of lead, and on the x-axis we have this week by week measure of developmental timing. And as you can see on the left of that dash vertical line, we're actually going into the prenatal period. And you will notice that, you know, at the beginning of this graph, the exposures are on the low side of things.
But right towards the end of the third trimester, the level starts increasing. And anyone who's familiar with the kinetic of lead in pregnancy, how it leaves the mother's bones and calcium stores and crosses the placenta along with calcium, will know that in the third trimester, as the fetus is growing very rapidly, lead transfer has been known to increase. The important point here is also that if you did a blood test anywhere in postnatal life, you would miss this period of high exposure. You would classify this child as a low exposure person, and in fact they have had a brief period of very high exposure.
Now, metals has been work. I've been working on metals and other groups have as well, and there's been a lot of literature. I should acknowledge the work of Needleman. And even before Needleman, people often forget that Linus Pauling won the Nobel Prize for analyzing metals and teeth.
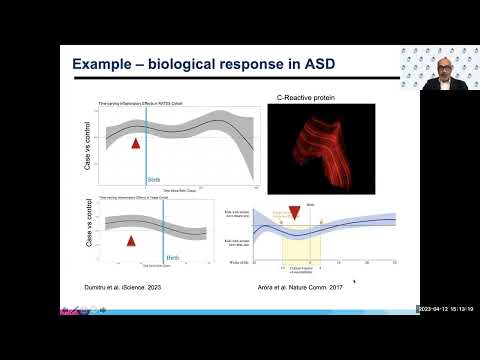
He was studying strontium and radiation. So analyzing metals in teeth has historically been a part of science. More recently we've been looking at other aspects, and one I want to share with you today is biological response. How does our body respond when we are exposed to an environmental stressor? And I'm going to use this one example of C-Reactive protein, a very well-established clinical marker of inflammation. This work was done at Sinai with Dani Dumitru, Christine Austin and other members of my team. What you're seeing here, and now you can actually visualize in a tooth growth rings, very much like that diagram of a tooth I showed you or picture of a tooth I showed you earlier.
And each of these bright red lines is a period of high intensity inflammation as measured by C-Reactive protein. And for the first time we can go back into fetal life and build a daily profile of inflammation using this technology. Certainly this offered us a powerful tool to ask the question, does inflammation play a role in a condition like autism spectrum disorder? So I worked with my colleagues at the Karolinska Institute, one of my closest colleagues there is Sven Bolte and the RATSS cohort is something he established many years ago. This is a case control study amongst twins. On the x-axis again, you're seeing the developmental time. Anything on the side is before birth.
What I'm showing here in this graph is a distributed lag model analysis of the CRP intensity in cases versus control. So if it's above zero, it's higher in cases. And what you see is this window just before birth and another window later around the one year mark where cases have more inflammation. Of course, I'm not going to, you know, accept this finding just based on one study than that's in Swedish twins. So we replicated this finding in a cohort in Texas that is in a population drive.
No, these are not siblings or related in any way. In this case, there was a limitation because of the difference in tooth type that our Texas cohort, the time dimension doesn't go into the first year. So we couldn't replicate the second window. But again, we see that both the prenatal windows or the prenatal window is seen in both studies as high inflammation in children prenatally who go on to then get diagnosed with autism.
As many of you know, the average age of diagnosis in the US is stubbornly stuck around four and older. As soon as I saw this study, and it was only published last month, it was a bit of an aha moment for me because it reminded me of a study that I had published many years ago, about five, six years ago, looking at just the metals. And this is a simplified graph of that and what it shows, if you look at this prenatal period, again, around that third trimester, there was a sudden drop in zinc and these were, again, the study was done in twins. So here we found that there is a metabolic disturbance of zinc in the third trimester where cases have lower zinc uptake at the same window we are seeing higher inflammation.
So there's a broad systemic dysregulation happening here. The fact that it's happening at this window is further bolstered by the work of others who are looking at things like air pollution, for example, and finding that third trimester higher pollution exposure increases the risk of the child developing autism. So this is very encouraging that we can now use this technology to find these critical windows. I want you to take a moment to consider if we exclude the time dimension. Let's take all of these graphs and reduce them to one single snapshot, like measuring blood somewhere in the postnatal period or even grinding up a tooth and just saying, we'll get one average. Just remove the time dimension.
Suddenly all these findings disappear. So I like to say these are not findings of concentration, these findings only exist in the time dimension and therefore timing is very important. That's the critical. I'll go as far as saying that time itself is the mechanism, not the concentration. This work is being led by Lauren Petrick, myself and our team at our lab at Mount Sinai, which is a HHEAR center where we are now looking at using untargeted omics.
So we can measure exposures. Many of the things on the left are, you know, chemical compounds that are quite toxic to us and we are studying through many avenues now. But we are also looking at things that, you know, are molecular building blocks and are biological response.
So this now allows us to build this pathway all from a single model. What is the exposure? What is your phenotype? How do you respond to it? For me, a much more satisfying view of molecular phenotyping. And it's all beyond what genetic or genomic based molecular phenotyping can do.
As I was doing this work, one problem kept propping up and it's a very simple problem if you really think about it. You cannot walk up to an individual, even a child and say, okay, hand me over a tooth. Sure children are going to shed 20 teeth but they will shed them when they shed them. So in a clinical setting, when a clinician needs to make a rapid decision, teeth are actually not a good matrix. And often they shed too late for autism, for example, they shed around the time where you've already made the diagnosis.
So they're a good research tool, but they're not such a good clinical tool. So we said, well where else can we find these growth rings? And a very easily accessible tissue is hair. Hair also grows with time, although my case that's probably not true anymore, but for most people, hair grows at about one centimeter a month. And suddenly we started analyzing the biology of hair and binding these rings and saying, well yes, this could be a short term biodynamic marker that can supplement the use of teeth. And I'm going to show you some very unique data and I want to acknowledge the source of this data. It comes from Rebecca Schmidt and Debbie Bennett from the University of California Davis.
And I also want to say it's not been peer reviewed yet, it's unpublished data. So consider that you are doing a study, it could be a natural experiment, it could be a randomized clinical trial. You have a start and an endpoint. And let's say you just take two measurements as we often do with blood testing. And I've just put these dots here, and you might look at this and think, well nothing exciting has happened. The level for this chemical, I'm going to use the example of tin, which is a toxic metal, is pretty flat.
But the reality is we are living with lots of black boxes. We don't know what this person has experienced before they joined the trial. We don't know what's going to happen to them, you know, in between the two measurements. So let me remove these black boxes and show you how from a single strand of hair, we went back two years in time, we have taken 2000 measurements.
Now resolution is about three to four hours here. We've actually gone lower, but that's not high throughput and this data hasn't been altered anywhere. There's no smoothing. You can see some points that clearly are outliers. And you'll see that if I had done just blood measurements, it's a completely inaccurate picture. A lot is happening in the middle.
There's various dynamics, peaks and valleys and there was in fact a high exposure of tin. These actually come from one of the major California fires. We are asked using this technology to study how those individuals who experienced multiple fire events, what are the environmental dynamics for them. We're also using this technology and I'll share some recent successes there to study autism. As many of you know, there is no molecular or there's no FDA approved molecular marker for autism.
It is diagnosed currently entirely on behavioral observations, which means that throughout the US, the average age of diagnosis is four years and older. And if you live in a remote area, it's as old as six years. But this is a real problem because we know the brain is rapidly developing in the first year. So any intervention would have the most benefit in the first year. Yet we have struggled to overcome this problem.
So we did a multinational study. I won't go into that details, but we use this novel approach that we call environmental biodynamics and we sure that we can even detect autism as early as birth, and up to the oldest participant in this study was 21 years of age. We then went on to show how we can actually, you know, correlate this to mechanistic finding using FMRI. And I'm very proud to share that we submitted this to the FDA and actually got breakthrough designation.
The FD has designated this as a technological breakthrough that can hopefully alter the way we clinically, you know, detect autism. And as it goes through the diligence process, eventually one day it'll become a clinical tool where a clinician can just collect a strand of hair non invasively and we can test it and we can provide some information that helps them diagnose whether a child has autism or not. I'll summarize by just acknowledging the HHEAR program. Mount Sinai has a HHEAR targeted and an untargeted core. We offer the tooth biomarker mainly for metallomics.
We do do organics in teeth. You know, we build robotic platforms that are quite accurate, however, they're not high throughput yet, so we are building the second phase of robotic platform that will be accurate and high throughput. I do want to mention that Christine Austin and my group has developed breastfeeding biomarkers as well, and I showed you the CRP work that looks at stress.
We have some hair metallomics work as well, but we usually use it only for pilot studies through the HHEAR program because it's not high throughput yet. With that, I'll stop 'cause I'm almost out of time. I just want to acknowledge my colleagues who I couldn't mention during the talk and also the various funding sources, especially the RIVER or R35 mechanism and the two HHEAR lab hubs that are at Mount Sinai.
And thank you again to the organizers for this opportunity. - Thank you so much Dr. Arora. So I'd just like to remind everyone that you can either put your questions in the chat box, or excuse me, the Q&A box or you may raise your hand and we will be able to unmute you and call on you. I don't think I'm seeing any, Dr. Arora, you mentioned the environmental biodynamics approach to environmental health research.
So can you talk a little bit more about the clinical implications for this? - Sure, sure. Thank you for that question. So it all started with this realization that, you know, and what I'm going to say is something that we all already know that the human body is just constantly changing. It's not just the environmental inputs, but our physiology is constantly humming.
We know that our cortisol levels vary throughout the day, if you look at body temperature, zinc levels and blood, nothing is static. You know, bones are turning over every day very slowly, but our neurons are firing very rapidly. When you think of clinical medicine as built around snapshot technology, most people like me will get one or two blood tests done per year and all our diagnostic, our health parameters are based on that snapshot. So we realize that there is a fundamental shift that is needed here. We need to move away from this idea of just snapshots and incorporate time. So environmental biodynamics says that we need a certain number of measurements to build a dynamic profile of our physiology.
Using some AI methods, we have found that we need a minimum of a hundred. Now how many of us are walking around with a hundred vials of blood samples? Collectively sequentially the answer will be very few of us. So it certainly asks us to look at things over time. It doesn't ask us to measure, you know, many, many things, but it asks us to measure many, many times.
And we start finding signals that are completely hidden in the time dimension. The autism diagnostic is one example of how we have come up with a clinically effective tool that doesn't rely on concentrations at all. In fact, we don't input any concentration data, we just input how things are changing over time and suddenly we have, according the FDA, even a world first very accurate marker of autism spectrum disorder. So that's the environmental biodynamics approach, something that prioritizes the time dimension. - Thank you so much. We actually have a question, and I can't really tell who this is from because it's not a full name, but I'll just ask it.
The question is, what biomarkers are you looking for in hair to indicate ASD? - Sure. So our first panel looks at various environmental inputs, include toxic metals and also dietary nutrients, for example, zinc and copper. We also look at how our body responds to them.
And so we look at various cycles, for example, the rhythmicity of all these inputs, which is a measure of how we metabolize them. However, we can measure many other things. We can measure thousands of, you know, organic molecules as well.
However, because our focus is on making this a clinical tool, we are always restricting it to the most information caring but parsimonious panel. So the smallest panel that gives us the most information because that will allow us to go to the FDA and, you know, get approval for this. We don't want any black box omics in a clinical tool. - Thank you so much.
I do not believe I see any other hands raised or any other questions in the box. So thank you again so much Dr. Arora for your talk. - Thank you. - All right, so our next speaker today is Dr. Steven O'Connell.
Dr. O'Connell has over 20 years of experience working in the sciences, from stream ecology to marine biology to analytical chemistry and toxicology. While obtaining his master's degree in marine science, he worked with the National Institute of Standards and Technology and has a decade of experience working in the private sector, in addition to several academic appointments.
He currently specializes in passive sampling approaches and holds two patents using the silicone wristband approach with a startup he co-founded, MyExposome. Dr. O'Connell. - Right. Thank you so much. I want to thank everyone for having me and it's a pleasure to talk to folks today about this relatively new technology and what's going on with wristbands and how can you use it, and what are best practices and what's going on with it. So that, as it was mentioned earlier, I work for a small business, MyExposome.
We're about nine years old at this point. We have a few patents. And really we started this to kind of get a bigger impact than maybe just relying on publications alone. It was also co-founded with Dr. Anderson at Oregon State University.
And really trying to get at these questions of what is a normal exposure, and normal obviously is relative to every kind of aspect, even life stages as Dr. Arora mentioned. But, you know, if you had a nice systematic way to get external exposures, you could start to prioritize chemicals and maybe even build a nice database where we can leverage epidemiological expertise and look at correlations and things over time. So what is this approach? The wristband are just silicone wristbands. You might have worn them for various fundraisers or other kinds of things that you've seen in the world.
And it's a very simple way to monitor organic chemistry. And that's typically what we're going to talk about today is just organic chemistry, not talking about viruses, bacteria, metals or anything like that, is primarily used for those organics, but a wide range of organics. And that's one of the reasons you'd use silicone because of that silica oxide backbone allows you to have some dipole-dipole interactions in addition to Van der Waals forces, which makes this a passive sampling material that's advantageous for polar, non-polar, lipophilic, lipophobic compounds. So you get a wide range of organic chemical exposure. The other advantage to use this is because because the silicone's worn against the skin, and we'll talk more about this in future slides, you're able to potentially capture multiple routes of exposure, and even potentially internal routes of exposure. So the original publication described this for research and this approach was back in 2014.
And I just put this out there, some folks have maybe have seen this before, but if you're not familiar with wristband approach at all, this is a early stage. We didn't really know if this passive sampling was going to be very sensitive or very advantageous to use in certain kind of environments. So we start with occupational exposure with kind of a known exposure to PAHs, hot tar asphalt roofing. And in this case we were really primarily interested in seeing if it was temporally sensitive or spatially sensitive.
And what we mean by that we had folks wear it for just a single day or an entire work week. And we said, you know, do we see a little bit of a dose response, you know, increased exposure next to a known source of PAHs, do you see increased load of PAHs and that is the case. And then along with that, you know, do you see kind of a fingerprint of exposure that's conserved among all these folks that are exposed to kind of a known source of exposure.
So in this case, on the right hand side, we have three different bar graphs and they have kind of a very similar profile of different PAHs and different ratios that are all conserved among one another fairly well. We did see a difference in magnitude in one of the participants in the top graph versus the other two. And it turns out when we went back later and had some follow up questions that person was a safety monitor and they weren't actually applying the hot tar asphalt to the roof.
So in this case you have a job site, people have different responsibilities, different roles, and the wristband was sensitive enough to kind of detect, those different responsibilities at this job site. So that was a clue. There were supportive evidence that, hey, maybe this approach might have some usefulness out in the real world and certainly in this case it did. So that was kind of the start. And then the kind of the, since then there's been some increasing publications used in the wristband approach since 2014.
All around the world, different research groups have kind of read that initial paper and then kind of done their own thing and doing all sorts of stuff, independent of Oregon State or MyExosome, folks are using technology, which is really cool to see. About 75 publications date at least, I probably missed a few here and there, there might have been a dip during COVID, but maybe that's just, you know, part of new technology and you're just going to have a little bit of variability over time. To extrapolate to 2023, you'd probably have over 20 publications, but who knows, you know, that's, you know, these things kind of wax and wane I'm sure. And this is a pretty strict definition of people using silicone wristbands as we describe in the original paper, not silicone as a personal sampling device and some other kind of form factor or not against the skin or it doesn't include review papers and other things like that. So kind of an overview of what's been done so far. If you look at those 75 papers, you know, what are the chemicals being targeted and kind of what are some trends that we kind of see out of all these publications.
A deployment time, so as Dr. Arora was mentioning, you know, time is really important of exposure, so you could potentially do as long as you'd like, but you know, that time weighted average and there's questions of uptake over time and things that you might want to capture. And there's also questions of like how onerous is to wear a silicone wristband for a long period of time. So typically most deployment times are three to seven days with the vast majority of publications kind of centered around seven days. And that's kind of what we recommend with folks that work with us that typically is a good kind of slice of life. It's long enough that you can get a lot of different types of chemistries with different uptake rates that might be slower to go into the wristband with enough quantity that you can actually detect it with your analyte method of choice.
But then also it's not so onerous that you know, it's hard to wear it for that long. Over 20% of the papers do some sort of biological sampling so they can compare kind of a urinary metabolite or a serum metabolite to the data that you get from the wristband and kinda see if that correlates pretty well or not. It's been used in over 17 countries.
Most of the United States, that's not surprising. Most participants have been adults, but over 20% of the time, people include children, adolescents, or even pets. And we'll talk a little bit about that in a minute as well.
Most settings are ambient, but over 20% are including some kind of occupational exposure. And typically what you see is firefighting, nail salons, agricultural settings, you know, those occupational settings where someone has a specific question in mind, whether it's pesticides, flame retardants or something else. As far as chemicals targeted, maybe no one's surprised that have has been in kind of exposomic space as far as organic chemistry. Flame retardants, and I know that's a big umbrella, you know, some folks are focused on organophosphate flame retardants or even still analyzing brominated flame retardants and some new flame retardants that are being used.
But PA flame retardants, big bucket I realize. But flame retardants, PAHs, pesticides, those are the top three. If we look at some of the other more interesting compound classes or newer compound classes, those that you see there at the end that only have like one or two papers, those are typically published in the last two years. So whether you're looking at metabolomics or phenolic compounds that are associated with disinfectants, especially during COVID, parabens, hormones or fluorinated compounds.
So those are kind of relatively new areas that we'll talk a little bit about later in the future slide. But we do see that we wanted to make a bigger impact and we did see that about 16% of the papers, one out of six or so are MyExosome clients. So that's kind of cool to see that we're having a little bit of an impact helping get the kind of technology out and seeing if it's a good fit. Sometimes it's not depending on what you're to trying to do and what your study objectives are. So when we consider this research, next couple slides are just kind of research highlights that I thought might be interesting for folks to think about or they might have this question already and we can kind of address it.
But, you know, does the wristband data correlate very well at all with these kind of internal biomarkers of exposure? And here we see a diversity of chemistry, nicotine, organophosphate esters and pesticides. You know, are they correlating pretty well with the wristband data? And in these cases they are. Not every chemical correlates well with biomarkers, but we do see a lot of significant correlations.
In the example on the far right, they actually had paired samples of humans, and in this case dogs. And what I found kind of fascinating was that every time you saw a significant correlation between the human wristband data and the urinary metabolite, you also saw correlation in that person's dog. So that was kind of conserved. So you know, using (indistinct) is certainly not new, but this is a suggestive evidence that you know, that that could be a viable approach in certain research questions. Overall, wristbands correlates similar or better to other technology.
They've been compared to active backpack monitors where you have a pump that's powered and someone wears a backpack and those wrist data is compared to that air only, kind of capture that route of exposure and you can easily back calculate the air concentrations used in the backpack and pump. And they've also compared with hand wipes. Interestingly, if you combine something that's kind of air only and then you combine like hand wipe that's dermal, when you put that data together, it looks, starts to look kind of like wristband data and that's suggestive evidence in all these cases. And certainly this is mentioned in these publications as well that the wristband is capturing kind of multiple routes of exposure, not just air but also dermal. And so along those lines, we applied for a phase one SBIR with NIEHS a few years ago and was awarded some funding. And we were interested in, well if we take that same silicone wristband and we prevent any kind of skin contact, you know, do you see a difference in total load or detections among these different devices? So in the picture on the left hand side, we took a wristband, it was worn normally, you know, against the wrist, it's the skin on the wrist against the skin.
And then we also took that same wristband, we put it into a tea ball of all things and wore it on the neck or on the wrist as well. So what we were interested to see is, well our hypothesis was for volatile compounds, things that are in the air, it shouldn't really matter where that wristband was or whether it was touching skin or not. And so we were kind of assuming that you would see no statistical differences between those configurations that that silicone on the body for volatile compounds and those are defined anything with a boiling point of below 250 degrees Celsius. For anything above that, we were expecting to see a difference because of these other papers had mentioned in our original paper we saw caffeine and we thought, oh that might be coming from the sweat actually. So we had a clue that this could be a case and for semi volatiles that difference of load could be more pronounced.
And certainly we do see that. We have significant differences in detections and total load. Detections are about threefold change.
And then total load is about, on average a sevenfold change on a given individual whether you have skin contact or not. And that also suggests that dermal exposure is quite pronounced and these are all compounds that were within the polymer, not on the surface of the polymer 'cause as part of our practices, we rinse the wristband off because we don't want to extract compounds that might be particulate bound on the surface of the wristband. So you rinse the wristband off, then you extract the chemicals and now you're more confident that the concentration you're seeing are all bioavailable.
So if you are able to, this is all part of this data set and all this is not published yet, but I just felt like sharing because I look at a lot of this data by myself, so it's kind of nice to share it with folks, but plans are to publish this. We just submitted a phase two of this grant last week, but if you knew, of all the chemicals that were found about 90 chemicals that you can see on the screen are all these little dots, those are each a chemical, representing a chemical. If you could assign them classifications based on those configurations, maybe you could start to model, you know, thresholds of physchem properties of, well at this point could you be confident that these chemicals are all coming from the air or at this threshold you're pretty confident that all these chemicals are coming from kind of a dermal source of exposure.
And then maybe you want to just try to consider everything. And so I made a multinomial logistical regression using all sorts of different inputs and tried to predict whether compound was air mixed or dermal. And I got that to about 85% accuracy.
But I would put caveats around that because this is only 30 people, so I'd want to do a much bigger study to start to build these models and confirm kind of the conclusions that we're seeing here. But as an example of what you could do with something like that, our boiling point prediction or threshold would've been about 220 degrees Celsius, where anything below that you'd predict that you'd have over 80% confidence that that chemical is coming from the air. And as I mentioned before, kind of the current convention, the line in the sand is about 250 degrees Celsius. So if you never knew anything about that, you're in the ballpark of that range.
And again, this is early data, so you'd have to make sure this is backed up with a bigger sample size. But very promising start so far that we can start to predict where these chemicals might be coming from and also show that, you know, the data is coming from more than one source. So if you were, you know, the next question you might have, well if you thought it was coming from the air, could you take that wristband concentration and back calculate that to an air concentration? And the answer is yes.
I mean, that's typically what passive sampling's been based on for decades. Typically passive samplers are used in, you know, environmental matrices, sediment or water itself, you know, streams, rivers, lakes, that sort of thing. And back calculating to water concentrations has been done for, again, decades. So the same kind of thing you could do for air. So if you're very confident that this particular chemical came from the air, can you back calculate to air concentration? So that's what we did with a DOD SBIR several years ago. And we used a vaporization system and a custom exposure chamber to put wristbands in there, vaporize the chemicals, expose them over a known period of time, and with static environmental conditions, which we've tried to mimic kind of indoor environments since over 80% of the time people spend their time indoors, just a general global average.
So you will have to make assumptions when you back calculate about temperature, humidity, wind speed, that sort of thing. Mostly temperature is a huge, huge factor so you'd want to get that right. But if you make those assumptions, yes it's absolutely possible to back calculate. And we were able to do some predictive models to predict some very key parameters, partition coefficients and a dissipation parameter that's useful to easily back calculate wristband data into air data. Similar to this, we're also working on back calculating to kind of an averaged surface concentration. So you can do the same thing for dermal uptake.
If you were very confident that the compound was coming from a dermal, you don't know whether that's clothing or carpet or some other kind of surface contact, but you could still say, well this is equivalent to an average concentration over this time period of, you know, 200 nanograms or whatever. So it's certainly possible to do that as well. So just like you do with the air, you could do something with the dermal. So that's kind of the current state that I wanted to highlight today. And I don't have too much time to go into all sorts of cool little aspects of the research, but it does seem to be, it's definitely a quantitative chemical organic exposure approach. It's a complex and comprehensive profile, which maybe is why it matches up with biological data as good as, or sometimes even better than in other external measures of exposure.
You can back calculate if you can, but obviously with the complexity of the data, you know, communicating this to participants if you're doing a report back or communicating the data bit to other researchers sometimes can be a challenge because of this complexity. And that's currently an active area research that people are doing some really good things about. So quickly here I just want to mention a few future trends and the things that I think are kind of neat and maybe worth pursuing. Obviously most people on this call, probably everybody has heard of perfluorinated compounds.
When I worked at NIST several years ago, this was a compound class I was familiar with and worked with in sea turtles measuring that along the east coast. So we have some internal data, again, not published up at the top that we did just, we had some real world samples, we analyzed over a perfluorinated method and we did see some evidence of perfluorinated compounds. And then here's a graph from a recent publication from last year where they saw several perfluorinated compounds in wristbands, in this case it was firefighters. So this is probably a pretty rich avenue of study and we're currently resubmitting a phase one SPR next month, to try to get some funding and do some research here, probably doing some best practices, how best to extract it, optimize these compounds and silicone and also understand kind of where the parameters are.
Like 'cause this compound, the chemistries in this compound class are pretty dynamic. So you'd want to know kind of, okay at this point, you know, you're better off using some other technology than a wristband for these perfluorinated compounds. But for this set, you know, this is a really good tool.
So that's kind of what that grant is about. And other people are obviously doing some really cool research in that area. Another future trend could be particulate and when I say particulates, I probably should have just said residues. 'Cause when you say particulates, everyone thinks of PM 2.5. And I'll be the first to tell you, if you're interested in PM 2.5, you should use other technology.
There's some great technology out there that does some really, really cool stuff and very accurate and all sorts of good stuff with PM 2.5. However, if you're considering something else, some other kind of like unique application, in this case there was a publication looking at gunshot residues, with the wristband, you know, the thought being that you have a repeatable surface area that's easy to wear. So that's kind of the reason why you might want to use the wristband in that case.
And certainly it's possible to rinse out the wristband, as I mentioned before. You could analyze the rinsate and then you could also extract the wristband for compounds that were absorbed inside and get kind of a dual look. Obviously if you're talking about particulates and residues, you know, you'd want to make it, make sure that you have a good fit for purpose for your research questions and make sure that, you know, you don't get confused with the bioavailability of some of those residues and stuff. But certainly this could be an area where wristbands could be used in the future. Another brand new area, these are pretty recent publications that people are looking at metabolism and associating metabolic pathways on the left hand side with wristband data. And on the right hand side they actually gave someone a vitamin containing niacin and they looked for niacin and niacin metabolites on the wristband.
So this is all getting at this, you know, leveraging a sweat metabolome that the wristband might be sampling. So now you're talking a little bit more about linking these kind of external measures with internal measures or directly measuring internal measures on the case on the right hand side where you're giving someone a, you know, potential source of exposure and then you're measuring that with a wristband. So there's a very new area that people are kind of considering, but I think it could be super fruitful for folks to pursue. Along those same lines, our internal measurements for drugs, hormones, other kinds of internal measurements. So we have some, the charts at the top are just some preliminary evidence that we have in house of detectable levels of progesterone and testosterone, but also ibuprofen. So just example of a type of compound that you might be able to sample.
But certainly you might think about blood thinners, another thing. So there might even be a, you know, clinical setting that the wristbands might be used for in that case. So that could be a really fruitful area. I'm considering writing a grant along these lines as well. And the examples on the bottom, those are just real world concentrations, not actual real world samples.
So they just took a relevant concentration of what these compounds are found in the sweat and then they said, well can you see that concentration in the wristband? And so they did see that, so they're going to pursue those lines of evidence in that case. With that, I'll just thank everybody and take any questions. And if anybody's going to ISES Chicago this year, Mark and I'll both be there and we'd love to say hi. - Thank you so much for an excellent talk. We actually do have a couple of questions. The first one is for PAH exposure assessment using wristband passive samplers, is it possible to capture PAH PAH interaction or PAH other organic matter interaction during human exposure? - So I'm not sure what that question is trying to get at and that's on me, that's not on the person asking the question.
PAH interaction, I guess you might be talking about, you know, can you sample other types of PAHs, like Alkylated PAHs or oxygenated PAHs. I mean, yes, you can. The answer to that is yes. You could sample PAHs and pH breakdown products as well using the wristbands. - Okay, thank you so much.
The next one is in compliance, whether or not the person wore the bracelet all day is compliance rather determined solely by self-reporting? Have you looked at any markers on the passive sampler that might verify the amount of time the sampler had contact with the skin? - That's a great question. So in the majority of these papers, and even in our own research, yes we're totally relying on people telling us, but I found people are very honest. We've even had, this is kind of what a, I'm sure this won't show very well, but we have, this is kind of where the wristbands come in, they come in these packages and we have a label and then some clients, some other researchers, they have other labels on the back where they say, Hey, if you took this off, just mark here, you know, have a checkbox or whatever.
So there's certain study design that you can have for protocols to maybe try to capture when people take it off. We do see that, you know, some people don't want to wear it during swimming. We've had ER doctors saying, well I can't have anything on my wrist during surgery. So that's a great reason. So in those particular cases, you know, you could say, hey, please put it back in the bag and tell us if you did. So people are pretty honest about it, oh, I've left it in my bedroom or I lost it for three days and then I put it back on.
So then, you know, like, what does that exposure mean? So fortunately people are kind of excited about it and it's a technology that's kind of easy to wear for the most part, but absolutely, you know, study design's going to be crucial if you want to be sure that someone wore it or had good skin contact the whole time. - Okay, thank you. What are the typical limits of detection of PAHs and pesticide of the wristband passive samplers? - Great question. So that's typically, yeah, I mean how low can your analysis, well, what's your instrument methodology, you know, that sometimes is usually the limitation. So if your instrument method could only see down to five nanograms, then you know, you could see down to five nanograms in the extract. So sometimes I can answer that by an instrument question, but to the askers point, you know, with all of these compounds, you have to get enough material into the wristband to get over whatever that threshold is.
So if your detection limits are 20 parts per billion, or you know, and that's typically what we see, just a short answer, you know, low parts per billion for PAHs pesticides is very typical when we do a screen for 1500 compounds, that you're sacrificing resolution for breadth. So that one's more like 200 parts per billion, you know, as a minimum. You want to be over that threshold and we still, you know, find dozens of detections at that level, but. And then VOCs are higher than that, you know, especially if you're looking at alkanes, well now we're talking about PPM levels. So if your detection limits below PPM, you're definitely going to see some VOCs in that scenario. So everybody's different.
Phthalates, you would not need a very low detection limit. You're going to see phthalates out the wazoo, organophosphate pesticides or flame retardants, excuse me. Those exposure limits or the concentrations we've see in the wristband are pretty high.
We're talking tens of thousands of nanograms or thousands of nanograms. So if your instrument methodology is, you know, below a hundred parts per billion, you're easily going to see that or a hundred, you know, nanograms per mil. Yeah. Okay. - We actually have quite a few more questions, so hopefully we to get to them all. So if for example, two people are exposed to the same chemicals, would the results from the wristband show the exact same chemicals or would there be individual differences? - Well, as we saw in that example earlier on, if someone was exposed to the same chemicals in that early slide, so we have a known hot tar roofing exposure. So they are being exposed to PAHs of a certain, call it kind of a chemical fingerprint, where you have different ratios, phenanthrene, naphthalene, whatever, yeah, they're seeing the same chemicals.
But as we saw in that graph, you might see differences in magnitude given that constant exposure because that person maybe wasn't near that. And then certainly in the firefighting cases, I've seen many papers where you have someone who's outside of the structure, in this case, like a structural fire, they have a different load than someone inside the structure. So same active site, same wristbands, same types of chemicals, but we're seeing differences. And typically, and there's been some other interesting research whereas someone looked at, well let's wear a wristband for this week and then give it a couple weeks and wear another wristband for a week and compare the data. And often what you see is a bigger difference between people than amongst a person.
And that kind of is reflected on, you know, like our daily lives we typically do the same things. We have the same kind of commute. We live in, you know, live, work in the same places. So, you know, those are kind of conserved, although those can change with, you know, you might move or whatever, but yeah, individual differences, yeah, they can often be pretty dramatic.
- Okay, great. There's two, I think two questions that are about things I think you did touch on on your talk, but they're just asking for you to repeat them. So somebody wanted to know about the other formats for the band, such as collars for possible like long exposures. I know you mentioned pet tags, I think the camera (indistinct). So maybe repeat that. And then somebody else wanted to know, could you please repeat the two quantitative measurements you were able to achieve with the vaporization chamber, partition, co-efficient, and one other one.
- Yeah, sure. So yeah, the two parameters that are really critical were partition coefficients because they're on a log scale. So if you have, oh, it's plus or minus 10%, well that's a hundred fold difference on a log scale. So you have to be pretty precise, you know, you want those estimates to be accurate. The other one was dissipation coefficient, little ke, and you can go to that paper in 2021 and look at the equations and you can see how that all fits.
I just didn't want to overwhelm people in just a 20 minute talk. But those are the two critical parameters that we use. And some people do a different way to back calculate, but they're all mathematically linked. Some people like diffusion coefficients and activity, chemical activity, but mathematically they're all the same. And then your other question was, sorry.
- Yeah, there are format, so you have the wristbands, I know you said- - Oh, format, yes. Right, right. So you can change the format, you can use silicone pet tags or dog tags.
They literally look like dog tags just made of silicone. It's just that when you change the form factor, you might be changing what routes of exposure you might get, right? So if you're wearing a dog tag that's not against the skin and against the clothing, now you're sampling the air and you might be saving that clothing. Often we find DEET in a tremendous amount of samples, ambient exposure like people and then in seasonality that you don't really expect to see deet, but it's there. And that is telling me that maybe the clothing is off gassing, maybe it's hard to get DEET out of clothing, I don't know. But we do see deet quite often all over the place.
That doesn't mean it's a bad thing, it's just there quite often. - Okay, thank you. The next question is, can you say a little bit more about what you think you are measuring when you report progesterone and testosterone in the wristbands? - What we think we're measuring. So what I was getting at that was, say you wanted to look at birth control measurements, right? Maybe there's certain measurements that you could use with a wristband or clinical applications where you want maybe a time weighted average over a week or a month instead of just a blood draw, like a single snapshot, what Dr Arora was mentioning. And I was asking my own doctor, like, is there any kind of blood measurements that would be helpful? And she goes, oh yeah, blood thinners, like that would be awesome to know more of monthly average than that. And this idea originated from devices, implants that people have to deliver birth control or other types of drugs.
We're just flipping that in reverse. So there are silicone devices implants that you can use for birth control or other types of drug delivery systems. But instead of delivering the drug, we're just trying to measure an internal measure of exposure. And those are just examples. So you're measuring the drug or you're measuring some kind of like biological compound. - The next question's two parts.
Wouldn't things like sunglasses or phone cases be other possible formats for collection devices? That's an interesting thought. And any chance for embedded temperature sensors could help in adjustments and back calculations. - Yeah, absolutely. So the first question about sunglasses, certainly, yeah, I mean you could do silicone shoelaces if you want. I mean, imaginations are limitless. You know, sunglasses, you know, you'd want to make sure that that was, you know, occupational exposure.
They're going to wear the sunglasses for the whole length of time. Depending on your research question, you can make it out of anything. Typically sunglasses, you're talking about hard plastics. I'm sure you could get some kind of silicone thing on your thing if you were just really focused on breathing zones. Like it's gotta be in the breathing zone, you know, you could certainly have something there if you felt it was important.
I don't typically always agree that, you know, the breathing zone and the wrists are so different when we're talking about the chemicals that we're talking about here, especially volatile organics, things that are in the air, that's it's not too bad. But yeah, and again, if you're using other kinds of materials and you're not using silicone, then you might sacrifice some of the chemical diversity that you're able to see because these other plastics are going to be more relying on Van der Waals forces and other intermolecular forces that are based on just kind of lipophilic compound. You know, you might limit your chemical breadth if you use different kinds of materials.
- So we have a couple more questions, but we're actually out of time. So I will be sure that you receive these, Dr. O'Connell and maybe you can provide an answer offline. So in closing, I do want to thank both of our speakers for their excellent presentations. I'd also like to take a moment to extend a special thank you to the organizers of the webinar series, as well as the HHEAR coordinating center for their support.
Thank you to all of you as well for your attention, attendance, and engagement in today's event. The slide isn't up yet about the next webinar, but before we close, I'd also like to announce that the next webinar in this series has been scheduled for May 24th from three to 4:00 PM Eastern. This webinar will focus on the chemical characterization of environmental samples and will feature presentations from Dr. Heather Stapleton, and Dr. Lee Ferguson of Duke University. To learn more about this event or to access recordings from previous webinars in the series, you
2023-05-09 10:51


