NCI Seminar 3D Imaging of Fruit Fly Neurons and Muscles
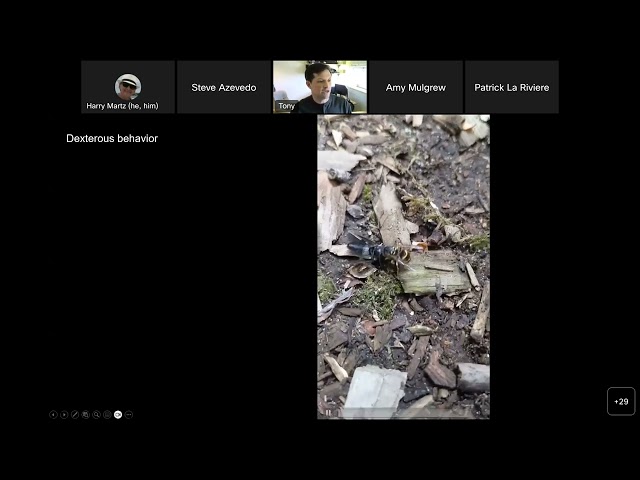
welcome to today's uh non-destructive characterization Institute seminar uh and I am going to introduce Steve azevideo who will be uh introducing the speaker for today Steve Aloha and uh thank you Harry for those who don't know me I'm a retired consultant having helped Harry form the non-destructive characterization Institute back in in 2014. nci's goals uh all along uh have been with our academic and government and Industrial Partners to understand the internal physical project properties of an object without causing damage it's more than detecting blips and signals and making images but it's really about characterizing objects using many forms of active and passive measurements like em and acoustic waves and particles along with physics-based analysis well our speaker today Dr Anthony Acevedo from University of Washington will talk about similar kind of work that he's doing in the area of Neuroscience they're studying neuron and muscle interactions um at the cellular level in in fruit flies using really novel Imaging methods that I thought would be interesting to this group um as well as invasive behavioral measurements like uh measuring the electronic spikes from a single neuron in a living fruit flies brain while just responding to a stimulus that's something you can't do very well with humans so if Tony's going to speak about their recently published work in collaboration with Harvard University and the European secretron facility in France and we both of which have these incredible uh very highly resolution unique 3D imaging systems that he's going to talk about uh he'll also talk about a another paper that's being submitted now for publication so we're hearing a kind of news on that if you have any questions for him we've asked Tony to set aside a few minutes at the end or you can type them into the chat we'll be monitoring that uh Dr azevedo's bio is in the seminar announcement so I won't go into and take up much more time um but he also happens to have been a at the Lawrence Livermore lab as a summer student and nif in the early 2000s so we'll give him some credit for the ignition shot in December a little a little bit of credit he help us also happens to be my son so uh so I'd love to hear about his work we thank you Tony for speaking with us today and welcome and take it away all right thank you Dad for the introduction um and thank you all for being here uh just checking to make sure you can hear me great awesome um yeah it's a great pleasure to be here and to talk about some of our work there's a lot of personal connections here uh Harry has known our family for a long time um we were just remembering his visits to Grenoble Grenoble France the European synchrotron research facility will feature in this talk but um Harry came to visit us while we were over there um and uh and like Dad said I spent some time at the lab after college before coming up here where I worked on Machine Vision and image segmentation problems um uh so like I said it's a great pleasure to be here but there's also scientific connections that I hope will capture uh your interests um and so I'm excited to present our recent projects using really novel Imaging uh and Machine Vision Technologies to develop comprehensive maps of the neural control of muscles in drosophila okay so I said our projects who is who is we um so I'm a research scientist in the lab of John Tuthill at the University of Washington um and uh this is his our lab um and uh this work is really thanks to a close collaboration that John has forged with Wei Chung Alan Lee a researcher at Harvard Medical School and Boston Children's and um in particular his former graduate student Jasper Phelps who was um instrumental in capturing these data sets that are shown up here and particularly on the left hand the left-hand one uh is thanks to some collaborations with Alexandra uh pakira knew a physicist at the European synchrotron research facility in Grenoble France um okay and then my role in this project has been to combine the information that we got from both of these uh data sets to address questions that our lab is interested in uh namely how the nervous system controls movement that is what we call sensory motor control and um I'm going to assume kind of zero Neuroscience knowledge but infinite intelligence uh and I'm going to spend a little time talking about what uh how we think about um the neural control of movement then I'm going to introduce these two data sets um and then talk at the end of the talk uh for a bit about what we're getting out of the connection between these two data sets okay so um you know fascination with how the nervous system controls movement really goes back to the beginning of imaging technology so um photography had been around for a little while but early experiments trying to invent cinematography or the cinema um uh were immediately directed at understanding movement so um Edward moybridge famously uh captured the running of courses and settled along standing bet that uh horses did not touch the ground during Galloping and he also captured images of himself and these early efforts inspired a pioneering physiologist in Russia named Nikolai Bernstein to develop his own cinemagraphic techniques and primitive or an early motion tracker actually nothing could really change nothing has really changed about motion tracking um until the past decade or so since his invention he used light bulbs to put to capture the movements of Craftsmen as they were swinging a hammer to hit chisels um and uh Bernstein was a a great thinker and like many great thinkers his real gift was being able to frame um uh uh frame a problem in an indelible way so that future Generations would be inspired by to study this more so one of the things he so here are two things that he thought about motor control so the first he thought of uh dexterous movement dexterity as kind of the Hallmark of uh the neural control of movement and he defined dexterity as motor wit or the ability to find a motor solution to any emerging motor problem so as an example um we often uh just consider you know trying to look at your watch you know we often take the other hand and move the sleeve out of the way so we can look um but if you're holding a cup of coffee you instead would kind of pop your sleeve and then look um and in both cases you're raising your arm and the first muscle you need to contract when you do that is your calf muscle actually so that you anticipate the change in the center of gravity As you move your limbs um so this illustrates that finding a motor solution anything even simple things requires a sequential contraction of muscles across the body so the second thing that Nikolai Bernstein um uh uh said that kind of captures this he really was fascinated by how the nervous system copes with all of these degrees of freedom how or how does it find a good way to solve any particular motor problem and just to put some numbers on that the human body has 360 joints it has 600 muscles and it has half a million motor neurons so motor neurons are the neurons that go out of the central nervous system to the muscles and this seems like a lot but it's a miniscule fraction of the um close to 100 billion neurons that are in power are our nervous systems and yet they're the critical link between the nervous system and the body and most of those motor neurons are in the spinal cord and it's protected by vertebrae whereas my brother used to call it the doctor uh he would call it the backbone mainly to annoy his attending uh people at the um during his residency his attending physicians um okay so since these early pioneering efforts to understand motor control we've made a lot of progress but we still don't understand fundamental things uh for example how activity in the brain makes it into the spinal cord and how motor commands to say move your arm or find your watch get translated into patterns of muscle activations and so our approach in the title lab is to look at an animal that's numerically at least a little bit simpler than humans and that's the fruit fly so here's a here's a fruit fly walking on a spherical treadmill so it's about a centimeter it's a foam ball about a centimeter in diameter and it's supported on a small column of air and the Fly is Tethered and moving this uh this spherical treadmill around um so flies are very agile Walkers they actually walk at a frequency they step at a frequency of 10 Hertz so they go quite quick this is slowed down uh over 10 times and you might say okay great this is Locomotion this is kind of a periodic Behavior but is this dexterity um and a naturalist would say that to find dexterous Behavior you have to you really have to go look in the in the wild and so here's an insect that is out in the wild my backyard here's a wasp that is navigating difficult terrain uh unstable objects that are about the size of itself it's walking backwards and it's simultaneously trying to do uh some other task which is to remove the head of this dead soldier fly and fly off with it okay so how does the nervous system control this kind of behavior um uh so flies and wasps have very similar nervous systems here's um the head of a fruit fly the brain is in the head and then it's connected to the ventral nerve cord or the VNC and the motor neurons that control the leg and the wings are located in the VNC they're dendrites where they get input is in these balls there's one ball per um per leg and then one ball for uh the abdomen and then another section where the wing motor neurons are um and we call these balls approximately we call them neural pills so I might mention that so these motor neurons get input from their respective motor neural pills and then send a cable out into the periphery that we call an axon an axon then targets a set of muscle fibers these Action potentials impulses electrical impulses travel down these axons they cause the release of neurotransmitter at the terminals which then go and attach to receptors in the muscle and open the receptors which excite the muscle and cause contraction okay so here's where drosophila really comes in handy you were probably familiar with the fact that the genetic tools for drosophila are um are very Advanced but here's the kind of thing we can do we can actually find fly strains in which we can genetically label individual motor neurons um and it would be the same motor neuron in each fly that we looked at so here are three different fly strains that label three different motor neurons that go to the femur um and you can tell that they're they range in size and these axons the the um the action potential would come down these axons and come to these terminals to cause contraction all right and then using these tools we can then do an experiment that's impossible invertebrates which is we can express a green fluorescent protein in um in the neuron and then using fluorescence microscopy we can Target an electrode to the cell body of the Soma or the cell body of the neuron also called the Soma and then measure the electrical signals in the cell um and so that looks something like this so here is a a fruit fly it's positioned on its back it's femur is glued down and it's tibia is free and then positioned on the tibia is this Force probe which we're tracking with this Red Dot um and you can see this muscle contraction uh that muscle contraction follows this uh here's the electrical recording from the cell you can see this this signal here we call it a depolarization the neuron reaches a certain threshold and then it fires this action potential waveform that's right there that action potential travels out the axon which we can record on a different electrode right there causes contraction of the muscle which you can see in the video and this twitch Force and then we can measure that same uh we can do the same experiment in multiple flies and measure the force produced per number of spikes so one two Etc in multiple flies and so this fast neuron here this big one produces pers for one Spike about 10 micronewtons at the tip of the tibia that's approximately the weight of the fly so that would essentially be like me curling my entire family with just a single impulse from one motor neuron but then what you can also see is that these three motor neurons produce a wide range of forces three orders of magnitude difference between this big axon and this smaller Axon okay um the fly is also still alive during these experiments so it can also behave on its own and that looks something like this so here is a a period where the fly is moving its own leg um and I'm recording from one cell in the cell body and recording the action potential of its separate identified cell um in the leg so you can simultaneously record the activity of both neurons as the fly is moving its legs around okay and this gives you a sense of what the motor what the nervous system must be doing in order to create movement it's controlling the temporal activation patterns of sets of motor neurons to create these movements and Bernstein was a contemporate or a contemporary of um of Norbert Weiner and around that time it was a beginning to be appreciated that control of movement required feedback which was led to the the field of cybernetics um but the exact circuits that are controlling and taking sensory signals and controlling motor neurons have remained pretty much a black box due to their complexity and scale and the best that we uh have done so far is essentially the experiments that I just showed you except blindly poking around to different neurons um and asking how their activity affects motor neurons and how external stimuli affect those motor knots any questions okay I just heard uh some input okay I will continue okay but since then um we now have tools that make it possible to do much better and I'm going to show you how we're addressing two questions here so the first thing we'd like to know is which motor neurons connect to which muscles that would um really allow us to start to parse these circuits and the second question is which pre-motor neurons connect to each motor neurons and the answer to both of these questions is two novel Maps the first one is called the projectome how do motor neurons project out of the central nervous system to muscles and the second map is called a connectome which is what are the connections between neurons within the nervous system okay so that's um an introduction to the kinds of questions that we're trying to ask in our lab and to address this I want to uh introduce you to this data set that we gathered at the um at the esrf and this is kind of why I'm here today um and to gather this data set we worked with Alexandra pakiranu who unfortunately wasn't able to um to attend this and tell you more about the instrument itself and we also worked with Peter claytons who has designed and built this instrument over the last several decades and that's that's uh this beamline ID 16a which is dedicated to these Nano CT experiments okay so most of the um most of the beam lines are located within uh the esrf building and so here each one of these are individual beam lines dedicated towards um uh x-ray imaging technology structures of biomolecules structures of catalysts all kinds of things and but these experiments require very coherent x-ray beam and so this experiment is located across the across the road down a very long hallway about 200 meters from the undulator source and at the end of that hallway is the experimental Hutch where the submarine as they call it is located and here's Peter and here's Alexandra um and the submarine is an ultra high vacuum container uh chamber uh that has cryo capabilities there's a scintillator designed in-house at the esrf um a couple to a fast readout low noise scientific CCD camera um the sample kind of is goes into this tube up here and then is dropped down into the submarine here's Alexandra loading sample into that tube and at the so here's what that pin looks like and at the very tip of the pin is our sample embedded in resin so here's a fly lag and here's the thorax of The Fly so to gather this info this um these data the um the instrument uses hard x-rays 33 uh Kev uh it's Focus the beam is focused with a pair of orthogonal lenses these Kirkpatrick mirrors down to a spot of 15 nanometers across which is the smallest that they've achieved at the at the facility so far the beam then diverges and passes through the sample uh the flux is around 10 to the 11th photons per second but that that was before uh the facility went through an upgrade which um I don't exactly know how much more but that's been since covid uh so it's it's significantly brighter now um okay so the the Holograms that you get on the scintillator are captured over here this is normalized to the empty Beam with no sample inside of it uh you could see some structure there um at each distance here uh it's the sample is rotated and uh 2000 Holograms are taken um the four different distances give you the phase information that you need to then compute the tomograph and the flylave is is kind of the fly leg is is the perfect size for this it's big enough um uh uh we can get a lot of resolution there but it's still small enough that we can capture the entire structure with only 10 of these tomographs so it took about three days to image the entire volume and Jasper was there um Imaging overnight uh the entire week so the initial results here were published a little while ago along with other data sets of neural tissue from Mouse cortex all right so if you stitch together all these 10 tomographs you get the following volume so here here out here I've Illustrated the outline of the exoskeleton of the fly and then you can see the tissues uh that we see as we go through this volume and for example um right here you can see the nerve carrying sensory information back to the nervous system the nervous system is here the VNC and motor axons out into the periphery and here you can see it meet up right there and then this is the nervous system right here and then these are cylindrical tomographs so you're watching kind of the edge of the cylinder as you're going through in z um so if we take a cross section through the femur of the leg you can see uh some of the tissue that we can observe um first this is the edge of one of these cylindrical tomographs that you see here and the stitching this instrument is incredibly precise in positioning the uh the sample so stitching this together is actually very trivial you just align them according to the displacement um that you needed for each sample or for each tomograph here in the middle we can see the nerve again carrying sensory and motor information uh This Big Hollow tube is a blood vessel which we call a trachea and flies these are individual muscle fibers and then we can also find the tendons these long black ribbons and then identify the tendons that each muscle fiber connects to to make up an entire muscle so muscles are made of individual muscle fibers which we'll see here um okay and then we can even see when motor neurons leave the nerve so this pair of motor neurons here is leaving the nerve right around here and entering this this muscle okay so using this non-destructive technique we were able to count the number of muscle fibers we see and so here here they are and they're colored according to whether we think the muscle is involved in pulling the flat forward during stance phase and those are blue or whether they're involved in extending the leg out to start a new Step which we call the swing phase and those are in orange um in yellow are the muscles that control the claw at the very tip of the of the Tarsus and then in red our muscles we don't actually know what their function is this joint that they seem to control is thought to be fused and yet there are muscles and there are motor neurons that innervate those muscles and so we have a biological mystery that we look forward to figuring out but we were also able to find where the motor neurons at least leave the nerves so we were able to count those exit points we weren't able to trace them all the resolution wasn't quite good enough but we were able to find that there were 67 motor neurons in this data set we were able to count the number of muscle fibers per muscle and in total There are 16 muscles depicted here then there are the two extra that control the Tarsus for 18 muscles total within the leg okay we've also used the data set to examine the sensory structure that measures the angle of the femur tibia joints and that's been published as a preprint and just recently went back in for resubmission foreign okay so that's that's the data set that's that's why I've been invited to talk to you guys um and uh uh but you can see so you can see the detail and the resolution that we can have now and we look forward to segmenting the tissues um uh better and perhaps using all of what we learned here to make uh neuromechanical models of of flies in the future which we can simulate in physics engines and things like that um what is missing here is connections from the nervous system onto the motor neurons and the resolution here is not quite good enough for that for that we need this we still need electron microscopy um and that's the only way we can see synapses the connections between two neurons and there's actually a synapse in this image here so it's right there um and uh if we zoom in a little bit more you can see the characteristic structure of invertebrate synapses they have this proteinaceous structure called a t-bar um and right around the t-bar are these little circles so the circles are synaptic vesicles they're small packets of neurotransmitter um and when this neuron gets excited these neurotransmitters fuse to the membrane right near the t-bar and release their neurotransmitter packets into the gap or the synaptic cleft uh where the the neurotransmitter diffuses over to receptors on the postsynaptic side and activates those neurons or causes a signal in the downstream postsynaptic neuron okay so it's a little bit hard to see here so maybe you can see it in this stack that I'm showing you over here so you can see this t-bar appear and disappear along with these little synaptic vesicle circles okay now some of you might be saying wait a minute stack I thought transmission electron microscopy was images of small slices um that is true and so I'd like to introduce you to one of the real radical Innovations of the last few years for Neuroscience which is serial section transmission electron microscopy um and so in this uh kind of audacious approach uh what's done is to fix the tissue and then to slice it in uh very thinly and then to keep track of each section serially and in this case um the uh the advanced the the advancement was to put this on a reel-to-reel tape which each one of these little um grids uh containing a slice of uh the tissue in in sequential order um so this was a technique that was invented by the lichmann lab at Harvard I was first applied to the the zebrafish brain the larval zebrafish um and then Davi Bach applied this technique to the drosophila brain so we have a a volume of the drosophila brain and then in this case Jasper and Whey applied it to The Locomotion circuits um that we're studying and each individual slice here took around 5000 images to look at so the one field of view was rather small you need five thousand of them to tile this entire slice and then about 4 000 slices to cover the entire volume about 20 million images about two to three terabytes of data to cover the entire nervous system of the fly at this resolution um how do you keep track of those slices you use what's called an ultra tome this is about a centimeter across and this is a an arm that slowly brings the tissue onto this Diamond Knife at the edge of a little well and then this grid tape is run through the well the slice floats off the slice is 45 nanometers thick gets picked up by the grip tape and then the next one comes um and uh and and it looks just like a camera uh the the device enters the tem beam this whole Contraption moves so that you can capture the entire slice and then you move on to the next one so whereas x-ray Nano CT might be considered non-destructive uh technique this is utterly destructive but um at the same time we can go back and image these slices again at higher resolution if we want to so this has been an amazing technological uh leap here but it's coupled with the um with the computation that's needed to actually align these slices as well so um convolutional neural networks stretch and warp each individual slice to match them um and then flood filling networks and additional cnns are used to then segment these data so this these methods are incredibly good now um they uh save orders of magnitude uh time um versus compared to manually tracing individual neurons which has been tried um but the segmentations are not perfect and so what you also need are collaborative and distributed distributive and distributed and interactive tools so that experts like me and other people in my lab can um can correct and manually proofread the segmentations that are initially created and so all those methods are described in these Publications finally um you need to find all the synapses in the volume and so another convolutional neural network is trained to detect these synapses and to predict which neurons are postsynaptic to that synapse and then finally you need some layer of annotation so that you can go in and say this neuron here is a motor neuron which it is this one right here is a motor um okay and so uh all of those Technologies were applied to our data set by the Wizards um in Zeta AI which is a company associated with Princeton University run by Sebastian sung a Pioneer in this field of connectomics um and also by researchers at the Allen Institute for brain science and the same group of people are applying these methods to a millimeter by millimeter by millimeter cube of mouse cortex as well and you can go find papers on that on preprints okay so using those Technologies we could turn an image like this into uh an interactive tool like this um so I've just interactively selected the neurons that were within that tiny little volume where that synapse was and you can see how many neurons light up the extent to which they cover the entire neuro pill here and also that some neurons carry information from other neural pills into this neural pill okay um we also were able to use uh you know from this segmentation we could count the number of neurons that are in there and so for the VNC there are 14 621 neurons approximately there's about 2 000 support cells uh or glia and there are 69 leg motor neurons that we found okay so these were the two data sets um that give us different levels of resolution for both the periphery and the central nervous system and both of these represent um you know decades worth of of innovation and um and you know the time has come for them and uh it's very exciting time for Neuroscience being able to track these these circuits down and the next step is to try to figure out what to do with them and so we're also excited to be in a position to explore what we can do um the first thing that we wanted to do was to connect these two data sets so there are no muscles in the serial section electron microscopy volume and there are no circuits that we can recognize in the X-ray volume so how do you connect these two um so the first thing to do is to try to figure out which neuron is which and so here are two neurons I've colored them orange and blue and the challenge really is to say um which one is which which muscles do these go to I should say um that this segmented volume this is the outline of the of the VNC from the EM data set the this nervous system was taken from uh an adult female so we call it the female adult nerve cord or fan C so I might refer to the fancy data set um we found 69 motor neurons on the left hand side we found 70 over on the right hand side and we can match them left to right so the extra one is um is a neuron that goes out and controls the very tip of the of the leg uh interestingly this side um the nerve on this side was cut very close to the tissue causing damage and causing the neurons especially the motor neurons likely to die begin dying over here so we found exactly half of the synapses onto the neurons on this side as compared to this side so I'm only looking at the left hand side here um as we speak though um more data sets more nerve nervous systems are being sectioned on the the um the ultra tone and are beginning to be imaged and these next ones will include both the brain and eventual nerve cord together so we will look forward to understanding how the brain feeds into these circuits okay so back to the question at hand you know which what makes the blue neuron different from the orange neuron um okay so to figure that out we took advantage of yet another impressive tool for drosophila it's a community tool um and that is 10 000 different genetic strains each strain labels uh different subset of neurons and then the creators of these tools then went in and created even sparser expression and created uh it took a hundred thousand images of um of sparse expression in different neurons so they look something like this where you can see an individual neuron to the point where you can recognize its morphology from uh these light these these confocal images fluorescence images and correspond and find the corresponding morphology in the fancy data set okay so then through Brute Force which is me actually uh I went through these hundred thousand images found 2 000 images of motor neurons and 200 of which look like this where there's a single cell that's labeled that we can can correspond so this single cell here is associated with a genetic label and we can bring this line into our lab from a Clearinghouse that's available it's in Bloomington Indiana where they keep thousands and thousands of fly strains and we can order them have them shipped in and then use a reporter line in this case green fluorescent protein um to label the the neurons that are labeled by this um by this label but it's labeling genetic strain so uh and Sue star in the lab creates these beautiful images of muscle fibers and the motor neuron innervation so we can see that this neuron um targets the fast ex the the excuse me we can see that this neuron targets the extensor muscle of the tibia and then we can go to the X-ray data set you can find the tibia extensor muscle um and we can locate the two motor neurons that enter this compartment and innervate this muscle which corresponds to two neurons in the fancy data set that um that look very similar to one another and that also corresponds with the number of neurons that we know from the literature innervate this muscle and they've been well studied in bigger insects like locusts and and stick insects so these are that one is more powerful than the other just like the neurons that I showed you before um and so we call this one the fast extensor tibia or the fetty and this one is the slow sensortibia or the seti okay so we ticked off two of the motor neurons two of the 69 motor neurons that we found uh and the other blue neuron that I showed you before is actually one of the neurons that I've been recording from in the past that we saw um responses from and so then we did the same thing for all the rest of the 69 motor neurons and assigned um them to their Target muscle and these muscles are either in the thorax and control the coxa or they're in the coxa and control uh the femur position Etc okay so now we have this this projectome by combining these two data sets along with other genetic tools available in drosophila and we have um we now know where that particular motor neuron goes we can then use the fancy data set to ask which neurons make synapses onto that and so that looks like this let me go back so here are individual neurons that all synapse onto that one extensor tibia motor neuron all right and then we can count up the number of synapses and we can do that for all of the motor neurons and we can create this connectivity Matrix where each column is is one of the 69 motor neurons and each row here is a different pre-motor neuron one of these different colored rainbow neurons as my daughter calls them um okay so um you can see from this connectivity Matrix that there is a fair bit of order here and I'll explain what the order is that we've applied to this um this connectivity Matrix so first of all I've grouped neurons together that have similar morphology so up here at the top are descending neurons and these are neurons that are actually located in the brain uh get input there and then bring an axon down into the ventral nerve chord next there are sensory neurons coming from the periphery then there are ascending neurons that send a process back up to the brain and likely report on the status of the of the body back to the brain there are intersegmental neurons which carry information from other neural pills that control different legs and likely coordinate inter-lim movements and finally the most numerous type of neurons are these local premotor neurons and the local pre-motor neurons come in all different shapes and sizes they make a wide range of number of synapses onto the motor neurons and then we also found that they tend to contact UM the same motor neurons or motor neurons that share a similar function so these are the two this is the seti and this is the fetty these both extend the tibia and all of these motor neurons make contacts onto both of the motor neurons and so we think of this as a kind of a modular structure between the the pre-motor neurons and the motor neurons that share a function and likewise these neurons here are examples that all target the tibia flexors that I showed you before okay so um the pre-motor neurons connect to these motor modules and form these modular circuits if we just sum up the number of synapses down the columns we can see that there's a wide range of number of synapses onto the motor neurons so the champion is the fetty that we've been talking about but Within These modules so these are all neurons that Flex the tibia there's a huge range in the number of inputs so this one receives almost 40 times the number of inputs is this one here despite that um each motor neuron receives a similar fraction of input from each of these different classes so about 60 percent of input to each motor neuron is from these local preamens about 25 is from these long range intersegmental or descending neurons and only about three percent is directly from Sensory neurons so this is possibly an underestimate because the sensory neurons were the hardest to reconstruct in this data set um but at the same time each sensory neuron seems to make most of its output synapses onto other neurons not motor neurons and so we think of this as a multi-layer structure we're looking at the last two layers the motor neurons they're pre-motor neurons the sensory information likely comes in is processed in a different layer by other neurons in the VNC and then that processed information is likely passed on to these pre-motor and motor circuits okay one last thing that we can do here that takes advantage of drosophila knowledge about drosophila is to determine whether a synapse is excitatory or inhibitory so excitatory synapses tend to make the postsynaptic neuron fire an action potential and inhibitory synapses tend to pull uh this neuron away from thresholds so you can think of it as negative and positive weights but it's impossible to tell by I whether a synapses and is inhibitory or excitatory but it turns out in drosophila that neurons that come from the same developmental origin as the as the um the organism grows it develops and adds more neurons to the circuits and if the neurons come from the same origin they tend to share the same neurotransmitter and the developmental Origins have been identified in drosophila and so here are a bunch of neurons that look very different in terms of their their processes but the connection back to the cell body all runs through a tiny bundle here and this bundle is called Hemi lineage 13A which suffice to say it's it's one of 30 different uh developmental lines and this one just happens to release Gaba which is an inhibitory neurotransmitter so all the synapses that you're seeing here are inhibitory so we can do that for all of the different developmental types and what we find incredibly is that um each motor neuron here each column receives about 50 of its inputs from cholinergic that is excitatory green synapses and about 50 of its input from inhibitory red or yellow uh synapses and in Gray are neurons that we haven't quite identified yet um okay so there's a one-to-one balance of excitation and inhibition onto each motor neuron despite the fact that this neuron here and this neuron here receive uh wildly different amounts This is 40 times as much input as this one the relative fraction from inhibition and excitation is identical okay so what else can that tell us about this Matrix so I mentioned before that we see these modules where groups of pre-motor neurons tend to make connections onto all of the the motor neurons that share a particular function but each row each each pre-motor neuron also makes synapses onto other modules and it turns out that it tends to be the excitatory neurons that do this um and uh so we measured the module preference which is the number of synapses within the module versus the total number of synapses here and the excitatory neurons have the lowest the lowest module preference meaning these excitatory neurons are the ones mediating these um uh these the control of two neighboring joints at the same time okay so that's uh then that's depicted here so an individual pre-motor neuron might connect to this module but not to this other module uh whereas in different pre-motor neuron might connect to the other module okay this is um the end of what I was going to show you about what we've learned from these two data sets as I think you can appreciate this is really just the beginning of what we want to do and at this point we're basically looping back around to this initial question now that we have these Maps what can we understand about how the nervous system controls movement um and as I've shown you today we have a lot of genetic tools that will now allow us to find individual neurons here we will know what their connectivity is and then we can do things like uh perturb their function during Behavior so going back to these initial studies of movement by moorbridge we now have using convolutional neural networks the ability to track in 3D the detailed kinematics of Lake joints um of a fly as it's walking on these these treadmills and perhaps soon even in Freely behaving flies and then we can acutely activate or silence these neurons and ask how those produce subtle changes in kinematics and Joint coordination leg coordination Etc okay so that's what I have to show you today I really appreciate the opportunity to um to talk with you all um and I have to acknowledge again all these incredible collaborators and the tahill lab so thank you again thanks thanks Tony a very interesting talk I have one question that is how long did it take to generate that map at the end you know it was just several years and all these people or yeah several years um so the initial data um the initial em data I think um it took about two years to get a volume that was correct uh you had to um like there was there were there were a number of different nervous systems that were fixed you first had to find how the orientation of the nervous system was right all that kind of prototyping so that you could get nice sequential slices all that prototyping took two years it took only a few months to slice it and then the Imaging takes a few months as well it was stitched together in steps so the first time it was stitched together it used kind of rudimentary um uh spline elastic techniques um and that was published in 2021 Network um and initially it was traced by hand all the motor neurons were traced by hand and it took two years to trace all the motor neurons um just the motor mounts and then it was redone so the the entire volume was re um realigned that kind of thing takes um you know several days uh now the segmentation takes only about a week to run and then the proof reading has taken a long time and we continue to do it so you can um you can Target the neurons that you're interested in we found all the pre-motor neurons to the all the neurons that connect to the to the motor neurons and we um uh set aside a dedicated month to um to proofread those and there you know the metric that we used was initially about 20 of the objects that are segmented are actually connected back to a Soma um but by the end of that month we had connected about 70 of the objects back to a Soma um so that gives you an indication of how quickly it's going and then after that it took another four months to get to the point where we have um this whole data set so each stage yeah has taken uh quite a bit of time a lot of patience right a lot of excitement too though uh do we have other questions I was wondering one of your um I'll ask I'm not going in one of your plots you had sort of these lines above it with that identifying Peaks I think in the data at first I almost looked like a barcode and then this one no it was much earlier than this one no oh oh yes yeah yeah yeah um you kind of had like a spectrum or some kind of plot and then above it it looked like you know I was guessing that maybe they were identifying Peaks yes we were looking at Peaks that's right I'm sorry uh I don't quite know how to exit out of this to go back you know no it's okay no that you passed it yeah that one yeah um yeah so um this is the membrane potential of this weak kind of slow neuron here and you can see these tiny little Peaks on top of that and those are the action potentials for this particular cell um the size of the action potential at the point where you measure it um has a lot to do with um the electrical structure of the neuron and so these slow these small cells have high resistance between the cell body and where we think the action potential is actually generated so for the signal to get back to the cell body it has to go through this this resistor which causes the the signal to become much smaller by the time it gets back to your measured device so you can see these big swings in the membrane potential but then on top of it you see these tiny little spikes you can you can detect them pretty well with filtering so there's I think there's a there's a low pass filter then there's a high pass filter and then there's a a shape matching procedure um and I mean that's what it is I wrote it but um and then you can detect these little these these Peaks here and so they're indicated as these tick marks here in this case a slightly different routine was um done to detect these Peaks but um yeah exactly um I mean one thing that I'll point out here um is that that mystifies us so this is one of the things we're kind of interested in is here you see that this neuron is the only one that's firing and that's when there's not much movement and then as soon as it begins to get faster then you start to see the recruitment of this second neuron which we know produces more Force and then the interesting part is over here you actually get more impulses in the um in the stronger one than in the slower one and so this kind of the shift from the slow one to the intermediate neuron is uh the job of these pre-motor circuits and we're trying to understand you know under what under what conditions you would go from recruiting the slow one first to recruiting the other one faster and which pre-motor circuits are involved so did um I I don't know if I misread maybe it's a slide before this that the slow the force output for the slow depends on uh if you send you know several pulses in succession is that the case uh yeah so yeah that's why yeah yeah so here this slow neuron is quite weak so we get we can't actually pick up the movement of the we can't detect the movement of the force probe in response to a single Spike here so this is an extrapolation back to one Spike um so it's usually spiking yeah so 2 30. do you know I guess this is maybe a kind of a wild question but do you know if the if the insect itself tries to you know vary the number of spikes to to to to to you know put more or less Force out or is this just kind of a very sloppy uh mechanism and sometimes you get 10 and sometimes you get 12 and there's kind of no in intention to to modulate that Force output um it's a great question um we don't know how precise they are yeah what you can see is that um when they're doing this kind of thing and when they're moving their their tibia around they almost never fire the very big one um the very biggest neuron is almost never it depolarizes so you see something that looks like this but then there's no Spike on top of it only in very rare cases does it Spike probably because it produces a ton of force and um if you accidentally recruit that neuron all of a sudden you're you know your walking is all thrown off right um how precise it is we'd like to know uh so I actually that's that's the next project using uh the maps that we have here um we're going to um study the circuits different circuits the behavior that we're going to um analyze is this learned behavior so we can actually punish the fly with an aversive stimulus and we can teach the fly to produce a certain amount of force on the tibia to turn off the stimulus and then the fly will actually produce that amount of force um and we got it working we received a grant to work on it and um and then we did all this stuff uh yeah we got the grant about two years ago and so now it's time to return to that experiment so we can answer that exact question okay great thank you okay again Tony I really appreciate it very interesting talk I love how you pulled all this data a lot of data fusion and a lot of stuff that we do as well as taking different measurement techniques fusing that data having this segment having the sort what's true what's not in that iteration and stuff like that is really interesting a very different scale right yeah a lot of signal detection and a lot of um uh um Roc analysis kind of stuff um I didn't mention a lot of that here but that will be in the in the paper that we're about to publish so or try to publish but yeah yeah no exactly well I learned a lot of this stuff at llnl um looking for yeah so I was working on a project that was Imaging um out of focus the Optics and out of focus and so you could see diffraction rings in these um in these images they were they they were supposedly um small cracks or um or uh uh you know problems with a lens upstream and the project was called finding rings in damaged objects uh in damage Optics which was which was Frodo typical lab stuff but yeah Roc analysis image segmentation true false positives Etc yeah was that with Laura uh kegelmeyer or Laura yeah yeah exactly and Barry okay yeah well thanks again I was really enjoyed your talk and and nice seeing you your sister and your um Mom and Dad thank you Harry thank you everybody really appreciate it thanks Tony thank you thanks for the invite
2023-05-31