NCATS NIH Translating Disruptive Technologies from Microphysiological Systems to Electronic Noses
Pat Brown (NIH): So now we're ready to begin day one, and our keynote speaker is Dr. Dan Tagle. Pat Brown (NIH): Can Dr. Tagle, can you please share your screen, so we can get started? Pat Brown (NIH): There we go okay so.
Danilo Tagle - NCATS, NIH: Okay. Can you see it? Pat Brown (NIH): Yes it's good, thank you very much. So Dr. Dan Tagle is Associate Director of the Office for Special Initiatives at the National Center for Advanced Translational Sciences, NCATS, at the NIH. Pat Brown (NIH): He leads several trans-NIH programs, including the NIH Microphysiological Systems program, also known as the Tissue Chip program, Pat Brown (NIH): the Extracellular RNA Communication program, a program called SPARC which stands for Stimulating Peripheral Activity to Relieve Conditions, Pat Brown (NIH): the 3D-Bioprinting program, and another program called ASPIRE which stands for A Specialized Platform for Innovative Research Exploration, and lastly, a new program called SCENT, Pat Brown (NIH): Scanning Conditions Using Electronic Nose Technology. Pat Brown (NIH): So these programs all involve coordination with other NIH institutes and centers, other government agencies such as FDA, DARPA, NASA, the VA, and BARDA, Pat Brown (NIH): which is the Biomedical Advanced Research and Development Authority in the Department of Health and Human services, services and also with the private sector.
Pat Brown (NIH): Prior to joining NCATS, Dr. Tagle was a Program Director for Neurogenetics at the National Institute of Neurological Disorders and Stroke Pat Brown (NIH): where he developed programs in genomics-based research and inherited brain disorders. Pat Brown (NIH): Prior to joining NINDS, he was a Section Head of Molecular Neurogenetics at the National Human Genome Research Institute Pat Brown (NIH): involved in efforts towards cloning genes for Huntington's Disease and other neurologic diseases. He has more than 150 scientific publications and has garnered numerous awards and patents. Welcome Dr. Tagle we're looking forward to your presentation today.
Danilo Tagle - NCATS, NIH: Thank you so much Pat for the invitation to speak Danilo Tagle - NCATS, NIH: this morning, and certainly to Danilo Tagle - NCATS, NIH: for the kind introduction and also for the other organizers for this meeting. So as Pat had mentioned, I'll be speaking a little bit more about NCATS and and some of the programs that I lead within the NIH. Danilo Tagle - NCATS, NIH: Just so just to give you a little bit of background about NCATS or the National Center for Advancing Translational Sciences, this is a newly created Danilo Tagle - NCATS, NIH: institute within within the NIH that started in late 2011. It's a bit different from the rest of the institutes and centers at the NIH and, in the sense that we are not necessarily disease focused Danilo Tagle - NCATS, NIH: or organ-specific in our mission, but our central mission is is essentially to catalyze a generation of innovative methods and technologies Danilo Tagle - NCATS, NIH: that will enhance the development testing and implementation of diagnostics and therapeutics across many human diseases and conditions.
Danilo Tagle - NCATS, NIH: And so, essentially we're looking at some of the the bottlenecks, and some of the areas where translation is being held back and and being able to innovate technologies that will address those issues. Danilo Tagle - NCATS, NIH: So in regards to the tissue chips program for drug screening or otherwise known also as Microphysiological Systems. The translational problem we're trying to address is essentially the the high attrition rate of drugs entering into clinical trial so as shown here only about 12% Danilo Tagle - NCATS, NIH: of drugs entered the trials and the high attrition rate is essentially because of high failure Danilo Tagle - NCATS, NIH: due to lack of efficacy about 55% and 28% can be attributed to have toxic effects in humans. Danilo Tagle - NCATS, NIH: Moreover, the average time it takes to develop a drug takes 10 to 15 years, but the average cost of $2.6 billion, including the cost of failures. Danilo Tagle - NCATS, NIH: So it's becoming clear that the current tools are being used for drug development that includes to the cell culture systems and and animal models are for predictors of human response, although they have been useful in the past they're not necessarily predictive of the human condition. Danilo Tagle - NCATS, NIH: And so that program goal of the tissue chips or microphysiological systems is to develop an individual platform Danilo Tagle - NCATS, NIH: that emulate organ physiology and function using human and animal cells and tissues with advances in stem cell biology microfluidic bioengineering Danilo Tagle - NCATS, NIH: that will result in the evaluation of efficacy, safety, and toxicity of promising therapies.
So this is just a rendering of what it looks like so we take a functional element let's say of an organ that such as the lung, which is the alveolus. Danilo Tagle - NCATS, NIH: represented, that in Danilo Tagle - NCATS, NIH: including the the biomechanics of the air sacs breathing in and out to vacuum channels and then being able to produce that in a chip, and eventually, we would like to represent each organ in the body with with Danilo Tagle - NCATS, NIH: functional element of the organ's into a human body and this human body on the chip system. Danilo Tagle - NCATS, NIH: I mean obviously there's going to be some some limitations in a census, as we go from 2D cell culture systems, to spheroids, organoids, 3D bioprinted tissues, and organ-on-a-chip or microphysiological systems, we are increasing Danilo Tagle - NCATS, NIH: physiological complexity, but at the same time, the limitation is that we suffer in terms of throughput so while do the cell culture systems can be can be high throughput in its capabilities, Danilo Tagle - NCATS, NIH: the microphysiological systems is at best medium to low throughput. Danilo Tagle - NCATS, NIH: Now, in terms of the use of non-human animal microphysiological systems, Danilo Tagle - NCATS, NIH: certainly, this can be used to reduce, refine, replace animal use and research, evaluate environmental chemical effects on wildlife species, advance animal health, such as in applications for veterinary research and and then all the subject of a recent Danilo Tagle - NCATS, NIH: National Academies workshop Danilo Tagle - NCATS, NIH: to bridge animal and human data that will allow more robust translation and drug development and regulatory applications of microphysiological systems data. Danilo Tagle - NCATS, NIH: Just to give you sort of like a general overview what microphysiological systems are essentially tissues or organoids Danilo Tagle - NCATS, NIH: that consists of multiple channel microfluidic cell culture systems as I've said that emulates major organ systems, so we have at least 10 major organ systems represented, the circulatory, the endocrine, GI, immune, skin, musculoskeletal, nervous, reproductive, respiratory, and urinary system.
Danilo Tagle - NCATS, NIH: And it takes Danilo Tagle - NCATS, NIH: essentially, representing the tissues, by taking into consideration to proper tissue scaffold, Danilo Tagle - NCATS, NIH: the proper cell types, the ratios of the cells amongst each other, so this is not just monolayer or single cell type but multiple itself that representing the particular tissues on the structure, the architecture of the tissues, spatial and temporal patterning, perfusion, Danilo Tagle - NCATS, NIH: by by including endothelial cells and vascular channels into the tissues, bioreactors that will allow the cells to survive Danilo Tagle - NCATS, NIH: and be in culture for multiple weeks, innervation, immune host response, functional readout, and computational design and and, obviously, it will take a lot of experts interdisciplinary collaboration taken place to make this happen, so we have Danilo Tagle - NCATS, NIH: teams involving material science scientists, cell biologists, tissue engineers, biomedical engineers, Danilo Tagle - NCATS, NIH: microchip fabricators and manufacturers, microfluidic experts, anatomy and physiology, pharmacology, and toxicology, Omics, regulatory science, and computational biology. Danilo Tagle - NCATS, NIH: Now, going back again into the lung, so this is a rendering of the long again to represent that we we go into the basic functional unit that along which are the air sacs. Danilo Tagle - NCATS, NIH: And this is the rendering of that.
We have the vascular vasculature represented showing here to microbeads, fluorescent beads coursing through those micro vasculature. Danilo Tagle - NCATS, NIH: This is work by Stephen George and then this this work by Jim Wells at Cincinnati. This is the gut enteroid that's been enervated and it's showing peristalsis. Danilo Tagle - NCATS, NIH: In this particular video, and then we also incorporate in line biosensing capabilities, whether it be microelectrode arrays or in this particular case optic genetic sensors Danilo Tagle - NCATS, NIH: that will show Danilo Tagle - NCATS, NIH: in this particular case, the parasites undergoing apoptosis, as a consequence of radical oxygen species generation so turning red to green. Danilo Tagle - NCATS, NIH: So this this are the actual chips themselves, so this is the chip the the lung on a chip.
This particular Danilo Tagle - NCATS, NIH: configuration distribute the vasculature on a chip this way the GI on a chip it's also versatile it also helps us to kidney and the liver. This is work by Teresa Woodruff Danilo Tagle - NCATS, NIH: generating the female reproductive system on a chip that would have Danilo Tagle - NCATS, NIH: the female reproductive organs, such as the fallopian tubes, ovary, uterus, ectocervix and coupled with the liver. Danilo Tagle - NCATS, NIH: And then, this is a commercialized version of a 10-organ chip commercialized by by a company a spin off company Hesperos that has multiple organs represented in this particular platform.
Danilo Tagle - NCATS, NIH: This is just a rendering of what has been funded by NCATS and NIH in the course of 2012 to 2017 essentially the proof of concept that we can Danilo Tagle - NCATS, NIH: generate multiple organ systems, all the way from you know the blood brain barrier, Danilo Tagle - NCATS, NIH: some of the liver and kidney and muscles and bone and so a number of investigators have worked Danilo Tagle - NCATS, NIH: tirelessly and in being able to come up with innovative solutions and and platforms, that will house individual organ systems and capture its salient points in terms of functionality and in response to various perturbations. Danilo Tagle - NCATS, NIH: So did just to show you an example, this is a data generated by Don Ingber's group at the Wyss Institute Danilo Tagle - NCATS, NIH: essentially showing the liver-on-a-chip, and so this is actually comparing the rat liver-on-a-chip with a human liver-on-a-chip in this in this particular case Danilo Tagle - NCATS, NIH: looking at the difference in species response within the rat and human. And so this is looking at the fialuridine which is Danilo Tagle - NCATS, NIH: an antiviral nuclear side analog was withdrawn during phase two clinical trial because of toxicity we're in about five out of the 15 patients that were treated Danilo Tagle - NCATS, NIH: actually, died and and the cause of death was a microvesicular accumulation in terms of lipids in the liver.
And so, looking at the treatment at from zero to 30 micro-Molars you can see that the rat shown by Nile Red staining do not show any Danilo Tagle - NCATS, NIH: noticeable effect, whereas in the human liver-on-a-chip, you can see that even at one micro-Molar are you start seeing Danilo Tagle - NCATS, NIH: vesicle accumulation as quantitated here and as show in this Danilo Tagle - NCATS, NIH: picture. Danilo Tagle - NCATS, NIH: Commensurately, one can also measure physiological Danilo Tagle - NCATS, NIH: biomarkers and this particular case albumin concentration, showing that, in the human liver-on-a-chip, there's a decrease in albumin secretion commensurate with the loss of hepatocytes, whereas in the rat chip, Danilo Tagle - NCATS, NIH: one one cannot observe such differences. Likewise, if you look at known liver biomarkers such as miR-122, glutathione S-transferase, and keratin you also see Danilo Tagle - NCATS, NIH: an increase in accumulation of those markers as a consequence of Danilo Tagle - NCATS, NIH: dose dependent treatment of fialuridine.
Danilo Tagle - NCATS, NIH: Not only can can we use the liver and the chips to measure toxicity, but can also lead to mechanistic insights into toxicity, so in this particular case, looking at the Danilo Tagle - NCATS, NIH: aristolochic acid metabolism Danilo Tagle - NCATS, NIH: and so, in this particular case, the liver-on-a-chip was connected to the kidney and and the neurovascular unit, the gut. Danilo Tagle - NCATS, NIH: But what is important in this functional connection the various organ system is that aristolochic acid, which is Danilo Tagle - NCATS, NIH: an ingredient found in Chinese herbal medicine primarily used for weight loss show that it's actually nephrotoxic but it's not known in terms of why nephrotoxicity happens, and so, in this particular case, if you just look at the kidney on a chip and treat it with Danilo Tagle - NCATS, NIH: 10 microMolars of aristolochic acid no nephrotoxicity is visible, whereas if the liver is connected to the kidney and and we see Danilo Tagle - NCATS, NIH: nephrotoxicity happening so what happens is that the liver Danilo Tagle - NCATS, NIH: metabolizes nephro- Danilo Tagle - NCATS, NIH: the aristolochic acid to form this alpha Danilo Tagle - NCATS, NIH: conjugated form Danilo Tagle - NCATS, NIH: which is turns out to be the one they active active metabolite that is nephrotoxic, as shown here. Danilo Tagle - NCATS, NIH: And by the live live/dead staining and, moreover, you can prevent the Danilo Tagle - NCATS, NIH: kidney cells from from dying in this case the proximal tubule cells by blocking the OAT4 transporter with probenecid as shown in this picture, so we show that the the Danilo Tagle - NCATS, NIH: aristolochic acid can actually be blocked Danilo Tagle - NCATS, NIH: and prevented from accumulating proof from probenecid and and that the toxicity is due to the liver metabolite.
Danilo Tagle - NCATS, NIH: We also then Danilo Tagle - NCATS, NIH: at the end of the proof of concept to show that the chips can be used for toxicity studies we then pivoted the program to see if we can develop Danilo Tagle - NCATS, NIH: these chips for disease modeling and efficacy testing as I've mentioned the high rate of drug development failures, because of the lack of efficacy so Danilo Tagle - NCATS, NIH: essentially, creating disease models that are more relevant to human condition, so this is an active program where we have a number of different activities ongoing from common to rare diseases. Danilo Tagle - NCATS, NIH: And also very complex diseases connecting multi-organ systems, such as in the Type-2 Diabetes. Danilo Tagle - NCATS, NIH: So, so a number of activities are being funded right now to develop various model systems for diseases. Danilo Tagle - NCATS, NIH: That the chips have also been Danilo Tagle - NCATS, NIH: used to respond to various health emergencies, about two-and-a-half years ago, the biggest crisis that our nation is was facing was the the opioid crisis still is a big crisis, and so we were fortunate enough to get some some money from through the HEAL Danilo Tagle - NCATS, NIH: which is the NIH Helping to End Addiction Long term, and so in this case five awards were given to model nociception addiction and overdose and then with the latest pandemic COVID-19 pandemic through the CARES Act, we were able to get Danilo Tagle - NCATS, NIH: money, through the CARES Act supplemental funding to use chips to model COVID-19 infection to understand multiple tissue organ pathologies, Danilo Tagle - NCATS, NIH: test candidate drugs and vaccines, understand immune response, and model complications from vulnerable and at-risk groups.
Danilo Tagle - NCATS, NIH: Moreover, there is now an active microphysiological systems Covid research working group, primarily, led by the United Kingdom National Center for Replacement, Refinement, and Reduction, the NC3Rs, with participation by NIEHS, NCATS, NIAID, FDA and the US Army. Danilo Tagle - NCATS, NIH: And, and some of the work that is coming out of this funding again this is work by by Don Ingber's group at the Wyss Institute showing how the lung-on-a-chip that which was just being used to study COPD Danilo Tagle - NCATS, NIH: was pivoted to now be able to work with Covid so in this particular case data in compared Danilo Tagle - NCATS, NIH: hot cells with infected with either SARS-CoV-2 pseudovirus or VSV pseudovirus particles and and then treated them with FDA approved drugs, so, in some ways antiviral compounds and shows, for the most part, that in in the 2D system Danilo Tagle - NCATS, NIH: you can you can see Danilo Tagle - NCATS, NIH: prevention of viral entry into the cells into the lung epithelial cells, but in Danilo Tagle - NCATS, NIH: in a 3D a human airway chip, which is more physiological and has the biomechanics Danilo Tagle - NCATS, NIH: of breathing it's only the Amodiaquine and and the Toremifene that showed effectiveness in preventing viral entry. Danilo Tagle - NCATS, NIH: So it shows that there's there's quite a big difference within how you would evaluate the effectiveness of a drug, in this case the antiviral in the 2D cell culture system versus a 3D culture system. Danilo Tagle - NCATS, NIH: In this particular slide I'm showing the new initiatives that NCATS is pursuing in addition to the disease modeling, we are also funding Danilo Tagle - NCATS, NIH: a number of studies that started just last year about 10 awards were issued for clinical trials on chips primarily to inform clinical trial design and implementation. Danilo Tagle - NCATS, NIH: And this is participated in by a number of institutes and centers at the NIH.
We also have a collaboration with NASA in terms of extending the culture life of this tissue chips from the usual 28 days Danilo Tagle - NCATS, NIH: to be able to expand the viability and robust function of this chips for a minimum of six months. Danilo Tagle - NCATS, NIH: And so, this is actually a contract solicitation that just got published last week, so if anyone is interested in being able to test the limits of the system and be able to to culture, it out Danilo Tagle - NCATS, NIH: for more than six months Danilo Tagle - NCATS, NIH: here's the site. And then lastly, NCATS is also very much interested in being able to establish an annual microphysiological systems scientific conference that will be international in nature Danilo Tagle - NCATS, NIH: primarily to facilitate collaborative research harmonization of regulatory standards and requirements Danilo Tagle - NCATS, NIH: on the training of new investigators in this area, and certainly the coordination within funding agencies and regulatory agencies Danilo Tagle - NCATS, NIH: and to foster sharing of data and resources, we were fortunate to be able to receive several meritorious applications with one award to be made in July of this year for the most Danilo Tagle - NCATS, NIH: scientifically meritorious application. Danilo Tagle - NCATS, NIH: Just to give you a brief background on on what the clinical trials on a chip program looks like again. Danilo Tagle - NCATS, NIH: This is more looking at how we can inform clinical trial and design and execution by way of establishing recruitment criteria. Danilo Tagle - NCATS, NIH: Being able to stratify the patient population in terms of who are going to be the best responders or non-responders as well as to develop clinically relevant biomarkers.
So in this particular case Danilo Tagle - NCATS, NIH: a number of the awards, as I mentioned 10 awards were made that will develop and validate rare pediatric and common disease models containing patient derived cells. Danilo Tagle - NCATS, NIH: And so, essentially, representing the patient demographics and then Danilo Tagle - NCATS, NIH: it's a 2-phase award, so the Phase 1 is to create the models and and then incorporate as many patient Danilo Tagle - NCATS, NIH: population so possible into several chips and then Phase 2 of the award would then be to test potential drugs for efficacy and safety in an assessment and clinical trials. Danilo Tagle - NCATS, NIH: And so, these drugs are either looking at perspective or concurrent trials that are happening and and in one instance being able to look retrospectively at a failed drug to see why why why that particular candidate drug failed in human trials. Danilo Tagle - NCATS, NIH: And so, these are the various projects that are being supported into clinical trials on a chip. It's going to be running for another five years or so, like I said it's Danilo Tagle - NCATS, NIH: it's a biphasic award it's so we're looking at non-alcoholic fatty liver disease, atopic dermatitis, dystrophin muscular deficient deficient muscular dystrophy, progeria, tendon inflammation, dementia, Danilo Tagle - NCATS, NIH: heart diseases, kidney, Danilo Tagle - NCATS, NIH: preterm birth, as well as prostate cancer.
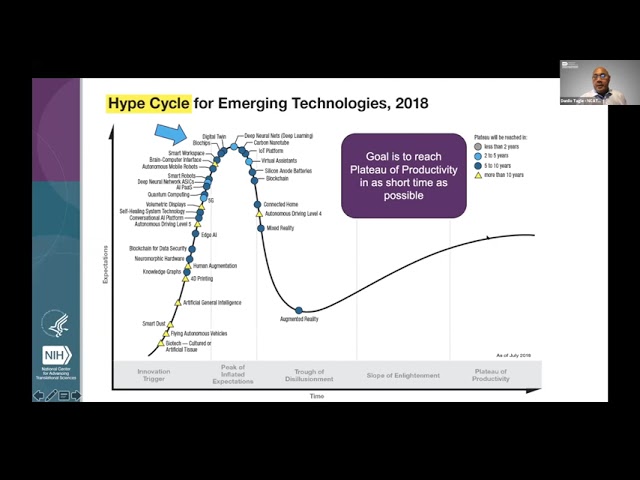
Danilo Tagle - NCATS, NIH: So these are the projects being funded at this point. Danilo Tagle - NCATS, NIH: And now, in terms of how what we're doing to increase the implementation and adoption or just technology. Danilo Tagle - NCATS, NIH: This is the Gartner chart for the Hype Cycle for Emerging Technologies which generally measures what it takes for adoption of a new technology. Danilo Tagle - NCATS, NIH: And for that to come into market, so this are sort of sort of the various technologies Danilo Tagle - NCATS, NIH: that are being watched by this investment group and so Biochips came into into the scene about 2013 and right around this Danilo Tagle - NCATS, NIH: point here and in 2018, this is where Biochips are, and so, essentially, the goal is to reach the plateau of productivity in a shorter time as possible.
Most new technologies takes anywhere from 20 to 30 years for adoption. Danilo Tagle - NCATS, NIH: And part of this is because of negative press that happens, supplier consolidation and failures, second generation products and services Danilo Tagle - NCATS, NIH: that happens, primarily in the in the in the area of the trough of disillusionment and then in the slope of enlightenment, those who survived Danilo Tagle - NCATS, NIH: the initial disillusionment will continue to develop standards methodologies and best practices and third generation products would be coming out and certainly most of this would be out-of-the-box products suites. Danilo Tagle - NCATS, NIH: And, and like I said, the goal is to reach the plateau of productivity, where there's high growth adoption phase. Danilo Tagle - NCATS, NIH: And then, greater than 30% potential end users has adopted the innovation and so what does it take to get to this face we have essentially Danilo Tagle - NCATS, NIH: three major challenges that's facing us, primarily, new technology must meet market needs so in this particular case, we must engage with the end users, which primarily would be industry and regulatory agency and then of course the end users, has to be able to use it Danilo Tagle - NCATS, NIH: easily, and it has to be cost efficient.
And so Danilo Tagle - NCATS, NIH: the next few slides will be addressing what we're doing to address this challenges. Danilo Tagle - NCATS, NIH: So in this particular timeline in terms of engagement with end users and multiple partners, I've mentioned Danilo Tagle - NCATS, NIH: the organs and chips, or tissues and chips program, actually predated the formation of NCATS back in December 2011. This was started with funding from the Common Fund to Advance Advancing Regulatory Science, which is a partnership with an NIH and FDA.
Danilo Tagle - NCATS, NIH: And then soon after NCATS were was formed we we've partnered with DARPA as well as with pharmacompanies to develop the toxicity models, as I mentioned in the past few slides. Danilo Tagle - NCATS, NIH: And then working towards modeling diseases, and in this particular case, working with NASA to develop models for accelerated aging models Danilo Tagle - NCATS, NIH: which I'll go into a little bit of detail in some in the next subsequent slides and, as I mentioned, we have Danilo Tagle - NCATS, NIH: various other disease models for nociception addiction and overdose for Alzheimer's Disease and other various disease models. Danilo Tagle - NCATS, NIH: But one of the key areas in terms of investment is building confidence in this platform. Danilo Tagle - NCATS, NIH: And so, NCATS essentially establish independent testing centers what we call Tissue Chips Testing Centers which are independent actually laboratories Danilo Tagle - NCATS, NIH: that have not worked with Tissue Chips before but would be looking to replicate the findings of the Tissue Chip developers and we also funded a database Center that will house Danilo Tagle - NCATS, NIH: all the data coming out of this program. Danilo Tagle - NCATS, NIH: What is what is inherent in the program is that the early engagement of stakeholders in this particular case, the US FDA has been involved from the beginning of the program and is currently still very engaged in the program and I'll Danilo Tagle - NCATS, NIH: have a few slides to show how engage the FDA is as well as Danilo Tagle - NCATS, NIH: pharmaceutical companies in this case, working with the IQ Consortium Microphysiological Systems Affiliate and the 24 or so pharmaceutical companies that are have a heavily interested in and invested in and seeing this microphysiological systems be adopted in drug development.
Danilo Tagle - NCATS, NIH: In terms of growing partnerships and investments beyond what NCATS is doing, as I mentioned, we have a number of partners within NIH, the Cancer Institute, the Cancer Biomimetics Program Danilo Tagle - NCATS, NIH: NIMH Nervous System Microphysiological Systems, NIDDK Modeling Diabetes, NIBIB ImmuneChip. Danilo Tagle - NCATS, NIH: NINDS' models for Alzheimer's Disease, Blood-Brain Barrier for NHLBI, Biomimetics for Infectious Diseases from NIAID, and then next generation of MPS nervous system and also Danilo Tagle - NCATS, NIH: in some ways a mark of a field that is matured sufficiently enough, is when the Center for Scientific Review at NIH creates a dedicated study section in this in this particular case CMT to review grant applications coming into NIH that will go into this dedicated study section. Danilo Tagle - NCATS, NIH: We have also partnered with NASA, as I mentioned, with through workshops and I've mentioned this contracts solicitation for Danilo Tagle - NCATS, NIH: establishing tissue chips that will be in culture for more than six months.
There's been a number of partners as well and presence Danilo Tagle - NCATS, NIH: in Europe. EUROoCS, Organ-on-Chip, NC3Rs, as I mentioned, hDMT, Japan AMED is also funding sources, Korea and China, in Australia, has also been Danilo Tagle - NCATS, NIH: working on on it various platforms and also work with partnerships with other agencies funding agencies such as BARDA, the TRISH, NASA Human Research Program, Danilo Tagle - NCATS, NIH: the EPA is primarily interested because of their mandate to be animal to be animal-free testing by 2035, the US Geological Survey, the VA, the Veterans Affairs, Danilo Tagle - NCATS, NIH: as well as the recent workshop that we have with the National Academies and ILAR on microphysiological systems for One Health and Comparative Medical Research. Danilo Tagle - NCATS, NIH: This are a number of companies that have either spun out or have been established centered around organs and chips and organoids technology. A number of these companies have Danilo Tagle - NCATS, NIH: gotten NIH support through small business programs, and it allows for the democratization of technology platforms which allows essentially pharma and other end users to choose from at least Danilo Tagle - NCATS, NIH: 20 companies for CRO-like services or the ability to purchase the platforms and and other consumables either in as well as the cells in this platforms.
Danilo Tagle - NCATS, NIH: In terms of building confidence I mentioned at the Tissue Chips Validation Centers. So in this case, we see the validation essentially in terms of three steps: Danilo Tagle - NCATS, NIH: physiological validation which is replicating organ function and structure, as well as response, and we do this with the training set up reference compounds obtained from pharma. Danilo Tagle - NCATS, NIH: And this validate physiological validation is essentially done by tissue to developers and has resulted in more than 500 publications as of October 2017. Danilo Tagle - NCATS, NIH: The second level of validation would be the analytical validation and this is done by the Tissue Chip Testing Centers which are independent sites and primarily looking at robustness, reproducibility, reliability, and relevance of the chips, and this is with a different set of compounds Danilo Tagle - NCATS, NIH: validation sort of compounds recommended by FDA and IQ consortium, as well as biomarkers and assays and and Tissue Chip Testing Centers Danilo Tagle - NCATS, NIH: that we have funded would be MIT and Texas A&M University and as I've said mentioned the microphysiological systems database that is housed currently housed at University of Pittsburgh. The final stage of validation would be the industrial Danilo Tagle - NCATS, NIH: phase, which were in industry and regulatory agencies start using the system.
Danilo Tagle - NCATS, NIH: And this is Danilo Tagle - NCATS, NIH: presumably done with proprietary set of compounds in a CRO type environment so in order to promote this we have encouraged our testing centers to be self-sustaining and so they have actually stand out. Danilo Tagle - NCATS, NIH: So the MIT is now a Javelin Biotech, which is a CRO business model and Texas A&M became the Tissue Chip Testing Consortium and and that should allow Danilo Tagle - NCATS, NIH: other stakeholders and end users to form collaborative activities with other end users to see what what platform is best use, and we can certainly continue to support the database to be able to make the data publicly available. Danilo Tagle - NCATS, NIH: And so, this is what the database looks like that this housed in the University of Pittsburgh so essentially it takes microphysiological systems data as shown here and also brings in preclinical data from animals and other 2D models clinical data, some of the Danilo Tagle - NCATS, NIH: drugs that have been tested in a lot of this systems through ChEMBL, and Unichem and DrugBank and then bringing other databases and essentially integrating this to be able to Danilo Tagle - NCATS, NIH: come up with analytical tools that will be able to show reproducibility Danilo Tagle - NCATS, NIH: and be able to do predict the safety and efficacy of drugs and have computational tools for disease modeling that is available at this and this database. Danilo Tagle - NCATS, NIH: One of the things that we have Danilo Tagle - NCATS, NIH: pondered about is is whether it's possible to model age related disorders, given this short shelf life of these chips and the months or years it takes to model age-related disorders.
Danilo Tagle - NCATS, NIH: And some of this changes, of course, or musculoskeletal changes, GI conditions, cardiovascular changes, respiratory changes, renal, Danilo Tagle - NCATS, NIH: declined in immune response, and decline in visual acuity and so can we model age-related diseases and initiatives can we develop drugs to mitigate age-related diseases and who are the right partners to engage? And so, as it turns out when when astronauts Danilo Tagle - NCATS, NIH: go up in space and get exposed to prolonged microgravity there are certain physiological changes that happens in their bodies that mimic Danilo Tagle - NCATS, NIH: accelerated aging. So within three weeks being exposed to microgravity they have upper body fluid shift, neurovestibular disturbances, sleep disturbances, bone demineralization and within six months, they have bone resorption, muscle atrophy, Danilo Tagle - NCATS, NIH: GI disturbances, hematological changes and then anything and anyone spending more than six months in space can undergo Danilo Tagle - NCATS, NIH: immunosenescence, renal stone information, but the interesting thing is that when when the astronauts return to earth there revert back within a few months to to normal physiology. Danilo Tagle - NCATS, NIH: And so having this background, we then NCATS partnered with NASA and the Center for Advances in Science and Space and the International Space Station National Laboratory to be able to see if we can Danilo Tagle - NCATS, NIH: address some of these Danilo Tagle - NCATS, NIH: issues of being able to model age related diseases under microgravity. So essentially, we have two goals, one is can we model age-related diseases Danilo Tagle - NCATS, NIH: by bringing them under microgravity on tissue chips. And so we have actually funded a number of projects that would look at immunosenescence, sarcopenia Danilo Tagle - NCATS, NIH: osteoarthritis, cardiac dysfunction, blood-brain barrier permeability changes, proteinuria and kidney stone formation, lung infection, bone marrow and immune response and gut inflammation.
Danilo Tagle - NCATS, NIH: And the second goal that we have is that, in order to adopt the technology here on Earth, I've mentioned one of the challenges is ease of use and cost efficiency what I said turns out, there is a requirement Danilo Tagle - NCATS, NIH: by NASA and in this particular case, the SpaceX Danilo Tagle - NCATS, NIH: in terms of what it takes for the payload to go up into space, and so we have to transform essentially refrigerator size Danilo Tagle - NCATS, NIH: incubator size, you know and like an incubator that houses, you know, in this particular case 24 chips, to be able to bring that up into the International Space Station NASA and SpaceX requires that it be reduced or miniaturized to from 48 cubic feet to 1.6 cubic feet. Danilo Tagle - NCATS, NIH: And so we were able to do this through partnerships with NASA SpaceX and some of the implementation partners space implementation partners TechShot, Space Tango, and BioServ, to be able to Danilo Tagle - NCATS, NIH: reduce this contraption to something the size of a shoe box. And so we were able to fund, as I mentioned a number of projects Danilo Tagle - NCATS, NIH: shown here in immunosenescence, osteoarthitis, blood brain barrier, proteinuria and kidney stone formation, lung infection, cardiac disorders, muscle wasting or sarcopenia, and gut in inflammation in the microbiome. Danilo Tagle - NCATS, NIH: And so Danilo Tagle - NCATS, NIH: there's been a number of to show that we were able to not only miniaturize but also automate the technology, because the astronauts are not Danilo Tagle - NCATS, NIH: necessarily scientists they wanted to requirements is that it's not only miniaturize, but it should be turn key technology, and so we were able to our first launch was in Danilo Tagle - NCATS, NIH: sending up the immunosenescence and the chip, and this was followed by subsequent launches through SpaceX 17 2021 and actually later today Danilo Tagle - NCATS, NIH: at 1:29pm, we will have a payload going up to SpaceX 22 that will study kidney stone formation at the International Space Station. Danilo Tagle - NCATS, NIH: Now switching gears this will be in terms of what FDA and pharma Danilo Tagle - NCATS, NIH: status on the uptake of microphysiogical systems, and so the FDA has actually formed an alternative methods working group, which has a way of web page presence in terms of Danilo Tagle - NCATS, NIH: indicating what their priorities are, they have a webinar series and alternative methods, this is participated in by various centers within the FDA by CDER, CBRE, and CFSPAN.
Danilo Tagle - NCATS, NIH: Center for Tobacco Products and Medical Countermeasures have also funded microphysiological systems for specific applications just looking at the effects of E-cigarettes and hookah on the lung. Danilo Tagle - NCATS, NIH: Moreover, FDA has established a new pilot program called ISTAND which Danilo Tagle - NCATS, NIH: is represents Innovative Science and Technology Approaches for New Drugs, which is in some ways a way to to fast track or to evaluate how new technologies as such as microphysiological systems can be considered as a novel drug development tools. Danilo Tagle - NCATS, NIH: And then microphysiological systems was also highlighted as one of the emerging technologies and focus of several sessions at the 2020 Global Summit for Regulatory Science. Danilo Tagle - NCATS, NIH: In terms of pharma, as I've mentioned we've been working with IQ MPS Affiliate.
Danilo Tagle - NCATS, NIH: It consists of 24 pharma companies Danilo Tagle - NCATS, NIH: representing Drug Safety, 3R's, ADME, and PK/PD. Danilo Tagle - NCATS, NIH: This group of Danilo Tagle - NCATS, NIH: representatives from various pharma companies have co-authored a series of publications Danilo Tagle - NCATS, NIH: on what it takes for industry to adopt the technology and the type of context of use that they would like microphysiological systems to be used for in particular for liver, kidney, lung, GI, skin, and cardiovascular Danilo Tagle - NCATS, NIH: platforms, so these publications can be found on their website and they have a series of other articles. Danilo Tagle - NCATS, NIH: soon to be published on disease modeling, ocular toxicity, oligonucleutides, cell therapy, Danilo Tagle - NCATS, NIH: gene editing, blood brain barrier, CNS, immune system, reproductive system and what what they're looking for in terms of how microphysiological systems can be can be adopted for use in these fields. Danilo Tagle - NCATS, NIH: So this our representation of some of some of the publications that already gone out the the eight or so publications that I mentioned Danilo Tagle - NCATS, NIH: that can be found at the IQ MPS Affiliate website. More importantly, the Danilo Tagle - NCATS, NIH: pharmaceutical companies have also started using microphysiological systems for internal portfolio decision-making and so these are just some of the organ systems being used Danilo Tagle - NCATS, NIH: heavily by industry, the type of areas that they're using them primarily with target identification, lead optimization, preclinical safety, Danilo Tagle - NCATS, NIH: preclinical efficacy, and PK/TK and and toxicokinetics as well and, and some of the publications have gone out, and these are some of the companies that have been working on these systems. Danilo Tagle - NCATS, NIH: Just in terms of what we're looking for metrics for success in this program so essentially the transformative outcomes that we're looking for the Danilo Tagle - NCATS, NIH: therapeutic areas of IND submissions containing chip data identification compounds with high likelihood of ADRs and compounds that are designated as safe, establishment of a standard for quality and predictability, and reproducibility compared with animal models and and and human studies.
Danilo Tagle - NCATS, NIH: Reduction in the speed of development of drugs and reduction in failure. Danilo Tagle - NCATS, NIH: Integration and use of organs or tissue chip as part of compound selection, reduction in various adverse events in human studies, refinement, reduction, and potential replacement in animal testing, industry and regulatory adoption of the technology. Danilo Tagle - NCATS, NIH: There's also been a recent survey of 15 pharma experts that forecast said that within the next five years or so, microphysiological systems, once adopted, into the pharma pipeline would say between 10% to 26% of R&D costs. Danilo Tagle - NCATS, NIH: In terms of driving cultural change, Danilo Tagle - NCATS, NIH: you know I, as I mentioned, we have an inclusive strategy for adoption and application, where we actively engaged funding agencies, regulators, industry academics, tissue chip developers to collaborate and work closely to address a number of issues. Danilo Tagle - NCATS, NIH: Some of the issues of course that that we work with will you know when rodent pilot studies still be required? Can non-human primates pharma Danilo Tagle - NCATS, NIH: PK experiments be reduced or replaced? What will in vitro-only approaches look like for entry into human testing? Danilo Tagle - NCATS, NIH: Will data into species still be required, or will tissue chip just be sort of like a third species requirement? What is the best use of omics-based readouts? And how can this be done with in silico approaches and data science? Danilo Tagle - NCATS, NIH: And so, these are just some of the things that we have done in terms of working pre-competitively through data sharing with pharma to the database working with the IQ Consortium regulators working potentially with with pharma Danilo Tagle - NCATS, NIH: to create through the ISTAND program so like a safe harbor type environment. Danilo Tagle - NCATS, NIH: And then being able to Danilo Tagle - NCATS, NIH: have expected transformative outcomes, including being able to enable prosecution of human only targets emerging from genetic genomics initiatives as you know Danilo Tagle - NCATS, NIH: more and more of therapies, whether it be ASOs, somatic cell gene editing are heavily focused on either human only targets or human sequence guided Danilo Tagle - NCATS, NIH: dependent strategies that will require human-like or representing human cells, at least in some of the initiatives also substantial time and savings in replacement of existing screens.
Danilo Tagle - NCATS, NIH: Being able to stratify patients for clinical trials and then, identify subpopulations that may need dose modification and predict the response of particular patients and risk for adverse events. Danilo Tagle - NCATS, NIH: Now switching gears. Danilo Tagle - NCATS, NIH: And going from microphysiological systems to mimicking dogs' olfactory systems, many of you know, probably know that dogs have about 220 million scent receptors Danilo Tagle - NCATS, NIH: whereas humans only have 5 million. Dogs have smelled receptors 10,000 times more accurate Danilo Tagle - NCATS, NIH: than humans, which means their nose are powerful enough to detect substances, a concentration, so one part per trillion.
Danilo Tagle - NCATS, NIH: Which means that it can detect a single drop of compound or liquid in a 20 Olympic-sized swimming pools. Danilo Tagle - NCATS, NIH: Dogs can also inhale up to 300 hundred times per minute in short breath, meaning that there are factors cells are constantly supplied with new odor particles or volatile organic compounds. Danilo Tagle - NCATS, NIH: So dogs in light of this...dogs have been trained to sniff out explosives narcotics for for search and rescue, and to detect diseases such as cancer, Parkinson's, onset of epileptic seizures or not narcoleptic moment, low blood sugar, migraine, malaria, and even Covid-19. Danilo Tagle - NCATS, NIH: However, there are limitations obviously they use of dogs for detection, there are some Danilo Tagle - NCATS, NIH: variation between breeds within breeds and between individual dogs, training can be expensive time consuming and it's not easily scalable, it has to be maintained, so that the dog doesn't lose their ability Danilo Tagle - NCATS, NIH: to detect these conditions.
Danilo Tagle - NCATS, NIH: And so NCATS then Danilo Tagle - NCATS, NIH: created a new program recently for scanning for conduct called SCENT which stands for Scanning for Conditions for Electronic Danilo Tagle - NCATS, NIH: With Electronic Nose Technology. So, our goal is to develop a non-invasive diagnostic device for rapid Danilo Tagle - NCATS, NIH: and accurate diagnosis, for a variety of medical conditions, and it will be using volatile organic compounds released through the skin, as the key substrate Danilo Tagle - NCATS, NIH: and to be able to establish a catalog of volatile organic compounds signatures unique for each disease, so we have an RFA that's out there, right now, screening Danilo Tagle - NCATS, NIH: and where we're looking for applications to be submitted. We've actually piloted this program Danilo Tagle - NCATS, NIH: a year ago, where we changed the C, Scanning for COVID-19. Danilo Tagle - NCATS, NIH: with Electronic Nose Technology, because we were fortunate enough to be able to get some red X [unclear] money to be able to pilot this program, and so we have issued for awards looking at volatile organic compounds from breath and skin, to be able to detect Danilo Tagle - NCATS, NIH: vox or volatile compounds from skin or breath that will pick up Danilo Tagle - NCATS, NIH: signatures of SARS-CoV-2, be able to Danilo Tagle - NCATS, NIH: acquire the signal through data processing artificial intelligence and machine learning, be able to then recognize those patterns and be able to come up with the diagnostics.
Danilo Tagle - NCATS, NIH: And so, with that I'll end with some acknowledgments primarily capable people that I work with my program managers Lucy Low and Passley Hargrove-Grimes. Danilo Tagle - NCATS, NIH: A number of program officers from various institutes and centers at the NIH or collaborations with FDA. Danilo Tagle - NCATS, NIH: With the International Space Station NASA and IQ and then for the SCENT program my program officer, Leah Tolosa-Croucher and Chariz Johnstone, as the program analyst. So with that I'll end and be able to take any questions.
2021-08-17