Multiple Sclerosis and the Spinal Cord
I'm very excited about this course, frontiers and the diagnosis and treatment of neurological diseases. It's been over 10 years ago when I was in medical school trying to decide what specialty to go into. I loved the instant gratification of surgery, the amazing part of delivering a newborn baby.
Really loved psychiatry too. So interesting seeing how the mind works in that way. But ultimately I did choose neurology, and at the time, none of my classmates, my good friends in medical school really understood why I choose neurology. Why wouldn't I choose something that was more lucrative, or had a specialty with a better lifestyle? To be honest at that time, our clinical rotation in neurology was limited. We didn't have as many treatments to offer to patients, and it was very frustrating seeing patients with these debilitating conditions. But what my classmates didn't understand was that the field of neuroscience is exploding.
I had just finished my PhD in neuroscience looking at synaptic mechanisms of how synapses were actually their own little modules. There are all these developments like channelrhodopsin was just being developed, where you could specifically control the activity of neurons and turn them on and off. We were using techniques in the lab to get specific molecules into the lab targeting different proteins, to turn those proteins on and off.
Then there's much more, the technology was becoming better, so that intracranial recordings and awake and behaving animals was more sophisticated with higher resolution. On the clinical side, I knew there was research looking at different ways to measure plaques in different substances and neurotransmitters in the brain. Then we're also learning a lot about mechanisms of MS.
Pretty much a new, almost a new type of neurologic diseases, a paraneoplastic syndromes was burgeoning and I just knew that the next few decades are going to be a boon for neurology based on the discoveries that we're making in the lab and I wasn't wrong. I haven't looked back from deciding to go into neurology. Today, we'll start off with the spinal cord, and then after that, we'll hear a presentation about multiple sclerosis from Dr. Joanne Guo. My talk will be brief, the anatomy primer. We'll start off with some basic definitions. Go over the components of the nervous system and then really focus on the spinal cord, its relationship to the brain, to the body, the bones.
Then we'll briefly just talk about some of the spinal cord disorders. I thought it'd be good to just go over some of the terms that you'll probably hear people using. Rostral really means anything that's close to the head, towards the head.
Caudal is towards the tail or to the back, where we would have a tail. Then ventral and anterior are referring to the front of the body. Dorsal and posterior are referring to the back of the body. Then also, you all, I'm sure know this already, but the basic component of the nervous system are neurons, which include a cell body, where there's a nucleus that contains the genetic material and where most proteins are made. The neuron really receives information from the dendrites, and information goes from the dendrites to the nucleus and then to the axons, which convey information to other axons or other neuronal cell bodies or dendrites. These are some of the major types of neurons.
In addition to neurons being the central unit of the nervous system, where electric information is transferred electrically and then chemically. There are a number of supporting cells and this is an oversimplification, but the main supporting cells are the astrocytes. They're the most numerous supporting cells in the brain.
They're star-shaped, which is why they're called astrocytes. They help with exchange of nutrients from the blood, and then they also help form this blood-brain barrier. They help control passage of substances from the blood into the cerebral spinal fluid. Then also, when these cells over multiplier or go awry, they're the underlying cause of one of the most devastating cancers of glioblastoma multiforme and such. Then we have apendymal cells which line the ventricles, the spaces within the brain, where CSF circulates and then also down into the spinal cord.
They make and secrete cerebrospinal fluid, and they have these little hair cilia that help move the fluid along. Oligodendrocytes are found in the central nervous system. They generate myelin that is a covering around the axons, and this allows for conduction. It serves as an insulator, prevents misfiring of signals and also allows for faster conduction transmission along the axon. In the peripheral nervous system, these cells are called Schwann cells. Then we also have these microglia, which are really derived from the immune system.
They remove cell debris and waste and help mediate any inflammatory response that's needed when there's pathogens that are in the brain. The other term you'll hear people using a lot is white matter and gray matter. Just as a reminder, gray matter is really the cell, but it's areas of the brain and nervous system where cell bodies of the neurons are concentrated. They're arranged in distinct layers in the cortex here, and the white matter are really the axons that are coming from those cell bodies traveling to deeper gray matter structures in the brain. So where neurons synapsing onto cell bodies and these deeper matters. You can see here gray matter is arranged on the outside of the brain, but within the spinal cord, it's actually inverse.
Gray matter is protected in the central part of the spinal cord and then the outer part is where the fiber tracts of the axons go. Now, focusing on the spinal cord. You see here it's really forms in different sections. It forms along with the bony spine. The bony spine grows a little bit faster and you could see that it ends up being much longer than the cord itself, which ends right about at L2 or the lumbar region.
But the bones continue on down all the way to the sacrum. You have the cervical portion which control sensation in the back of the head as well as in the arms. There is thoracic nerves which control sensation and muscles in the trunk and the chest and the abdomen.
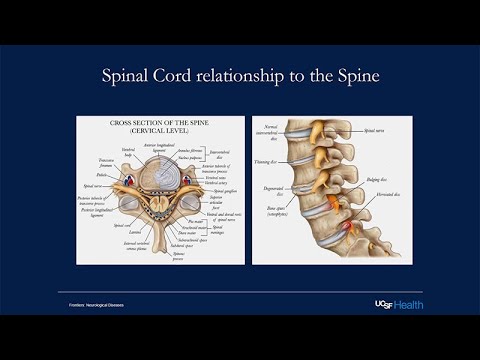
Then the lumbar spine, which controls movement in the legs. The sacral nerve roots also go to the a sacral area, the perineal area, and help to control urine function, bowel and bladder function. This is the spinal canal within the spinal canal.
This is across, this is like a cut of the spinal cord and I've switched it backwards because this always confuses me how they orient things. But this is posterior and this is anterior. This is also posterior here and this is anterior here. Then you can just see the organization again, the gray matter here. Then in the anterior section you have more of the ventral roots, which are motor roots.
Then posteriorly are the sensory roots that are coming from the dorsal root ganglion where the sensory nerves are coming in here. The spinal cord is more than just a conduit from the brain to the body. There's a lot of different cells and different processing units in the spinal cord itself. But ultimately, we think of motor signals and sensory signals originating from the brain and sensory signals coming into the brain. For example, this is a classic motor pathway. Once you decide to move a muscle, that neuron in the primary motor cortex of the frontal lobe is activated.
That goes down. That neuron here sends its axon down through the top of the spinal cord where it crosses over onto the other side where the right side of the brain controls the left side of the body and vice versa. Then goes into the corticospinal tract, this is called the upper motor neuron. The upper motor neuron synapses onto the cell body of the lower motor neuron in the gray matter of the spinal cord.
Then this neuron goes directly to the skeletal muscle to cause it to contract. In the opposite direction we have the sensory pathways. For example here, vibration, proprioception, knowing where your limb is in space and light touch comes in from the skin and the extremities. It goes through the dorsal, so the posterior and the back root ganglion, where there's, usually a pseudo-bipolar neuron that then sends its axon. Here, goes up again in the white matter tracks and the posterior columns and back. Then it crosses over in the bottom of the brainstem where ultimately it goes up to the brain, which is the primary somatosensory cortex.
That's right behind the motor cortex in the parietal lobe. We have, sensation is pretty complicated. In addition to vibration, we also have pain and temperature. Sensation which goes through different nerves and actually go through completely different pathway.
These pain and temperature fibers also go through the dorsal root ganglion but then they cross pretty quickly over to the other side into the white matter track called the anterolateral pathway, spinothalamic tract. Then once it crosses, it just follows up into the thalamus, one of the deeper parts of the brain, and then finally to the somatosensory cortex. At each level where these sensory fibers are coming in, they go to a specific dorsal root ganglion of the spinal cord. We can actually nicely plot out which part of the body, the region of the skin that's receiving the sensory input.
That's why if you do come to a neurologist, we're testing sensation at these different levels because we're trying to figure out if the sensation is coming from a specific nerve root or if it's more general, coming from the brain, or even from nerves which is different. You can just see at each level, for example, sorry, the colors don't align, but let's see very commonly. L5 is right here. You can see it enters the spinal cord there and then follows those sensory pathways that we discussed.
I thought this is just a nice illustration of this spinal cord doing a lot more than just being a conduit of information. For example, when we're testing reflexes, we can test specific levels of the spine. Here we are testing the triceps tendon.
When we stretch that tendon, there are these sensory receptors that detect stretch. They go directly to the spinal cord again through that dorsal root ganglion into where the cell bodies are and actually synapses on two different neurons. One neuron, motor neuron goes to the triceps muscle to cause it to contract. Then the other synapses onto an interneuron. This neuron then synapses onto the biceps tendon, which is opposite to the triceps, to allow it to relax.
The biceps relaxes, but the triceps contracts so you get the reflex. One of the most important things when thinking about the spine is its relationship to the spine itself. These are nice images looking at how the spine is related to the bony structure around it. It's very well-protected. But you can see that a lot of problems can come out when these disks, which are what cushion the spinal cord as we age, they tend to bulge or shift and then that can cause compression, not only backwards into the spine, but it could also cause compression here and bulge out. Bulging disc can hit the nerve roots.
This depending on where it's hitting, can cause pain, it can cause weakness, it can cause numbness as well. When some of the most common diseases of the spine we see are really compressive based on degeneration and arthritis of the spine. This happens when there's bulging disc or when there's factors or when there's disk osteophyte, so little bony outgrowths of the spine that can really damage the spinal cord or the nerve roots as they come out. Then of course, trauma can also damage the spine.
Other things that can affect the spine are conditions like transverse myelitis, which can be caused by multiple sclerosis, we'll hear about, as well as other auto-immune diseases and inflammatory diseases. Tumors do affect the spine, both metastatic and primary tumors. Infections that cause abscesses or some viruses can directly go to the spine and affect the neurons in the spine. There are neurodegenerative diseases that affect the spine such as ALS, and which affects both the upper motor neurons and lower motor neurons and then there are disorders of vitamin deficiencies or toxic conditions which can disrupt signaling preferentially in the spine. A little bit more rarely, just like we get strokes or ischemia in the brain, there can be strokes or spinal cord infections of the arteries going to the spine, as well as vascular malformations that can really affect spotting the function.
That's really it for my anatomy primarily, these are the main references that I took the pictures from. With that, I'll just introduce Dr Joanne Guo having worked with her on several cases now, I know that she's just an incredible, amazing empathetic clinician. She's a fantastic speaker. She presents at our annual recent advances in neurology course, which is geared towards physicians really all over the country. She is an integral part of the scholarship and excellence. UCSF multiple sclerosis in neuro inflammation center, which is housed here at the Ohio.
Just a little background about her. She graduated Suma Kumlati from Ohio State University, where she also did medical school and then went on to do a residency at the prestigious Columbia University Medical Center in New York. Luckily, she moved out to the West Coast of your fellowship in immunology here and joined our faculty afterwards. I'm really excited for her to start off our course. I couldn't think of a better person. She'll talk about multiple sclerosis, which has been one of the really transformational areas of neurology where our scientific understanding and drug development has really come together to make a huge impact on changing the course of patient's life.
Thanks, Joanne. Hi everyone. Thanks so much Maggie, for that wonderful introduction. I would say all the same things about you as well. As Maggie mentioned, I am a clinician in our UCSF multiple sclerosis and neural inflammation and center.
I do primarily see new diagnosis and chronic management of people living with multiple sclerosis but we also have a broader neuro immunology neural inflammatory clinic where we see other associated diseases and also other types of autoimmune inflammatory diseases that affect the central nervous system. But today I'm going to be talking primarily about multiple sclerosis. Many of you here may have heard of this disease or at least have some idea of what it is. Very broadly, it's an auto-immune disorder of the central nervous system, meaning it primarily affects the brain and spinal cord.
It's a type of condition that we categorize broadly as a dean myelinating condition. As Dr Waung mentioned, myelin is that fatty tissue that is around nerves and protects nerves and help basically nerve conduction run faster. Most classically multiple sclerosis is characterized by areas of inflammation and demyelination. Injury to this myelin and oftentimes we can see this injury on magnetic resonance imaging, also called MRI.
We can visualize this imaging with testing that we have in the hospital, in the clinic. But it's also a condition that over time can also injure the axons of the axons and the nerve cells themselves and sometimes we can't always see this as well on imaging. Over time, someone who's had very longstanding multiple sclerosis we may see this as, atrophy or shrinkage of the brain and then oftentimes, it's something that's better visualized, actually postmortem or after someone passes away and actually looking at the pathology tissue.
Before we had the ability to do MRIs and to image people the way we do now, when this condition was originally described, they really could only look at it after people had passed away and they could actually see these areas of information and plaque and scar actually on people's brains and spinal cord and that's how it got the name multiple sclerosis, literally meaning just multiple areas of scar. This is one of the most commonly conditions that affects morbidity and mortality in young people. It usually presents in young adults age 20-30 years old has a prevalence rate that ranges from five to 300 per 100,000 and tends to have higher rates at higher latitudes. The development of MS has been associated with a few different things. We're still not sure the exact etiology that we don't think there's one thing that causes MS, but it's probably a lot of different factors that come together and there's different associations that we've seen with it.
One of the strongest associations we've seen with it is actually less sun exposure and low vitamin D levels, which is why we think we see it more in higher latitudes. At certain genetic factors, certain genetic alleles have been associated with the development of multiple sclerosis and carriers of these types of alleles that probably are at higher risk for developing MS, childhood obesity, cigarette tobacco use and something that has been getting a lot of buzz lately that we've recognized in the MS community for a long time is the likely association with Epstein-Barr virus and the development of MS and other types of potential viral triggers as well that may lead to the development of MS. Again, it's probably not one factor, it's probably multiple things coming together that leads to the development of MS and we're still trying to understand that fully here at UCSF.
The clinical presentations of multiple sclerosis, there's different phenotypes or different clinical presentation to you guys will probably hear about. The most common type is called relapsing, remitting Multiple Sclerosis, which is characterized by recurrent neurologic symptoms that can last weeks to months, but usually improve or at least partially improve over that timeframe. The types of clinic we call them clinical attacks or relapses that people may have. Maybe things like vision loss, usually caused by inflammation of the optic nerves. We call it optic neuritis; itis meaning inflammation, can also, if it depending on where it affects in the brain or the spinal cord, you'll probably have focal neurologic symptoms related to that. As Dr Waung mentioned, different parts of the brain and spinal cord can map out to certain functions or dermatomes, weakness, etc.
Depending on what part of the brain or spinal cord is affected, you'll have certain types of neurologic conditions. Some examples of this could be weakness in the arm or weakness in the legs. Could be bladder dysfunction with weakness in the legs, any combination of it. The types of relapses are a little bit unpredictable and can also occur sometimes months to years apart, which can sometimes make the diagnosis really hard to make. Different from a stroke where symptoms usually come on. We usually say like snap of a finger.
All of a sudden, there's all these neurologic deficits that come at once with multiple sclerosis, it tends to be a little bit slower. We call this instead of acute, we call this march sub-acute onset where things may develop over hours or even days and then peak within a couple of weeks and then get better sometimes over a couple weeks to months at a time. Sometimes with treatment which I'll talk about in a second with certain treatments of relapses, sometimes people can get better right away.
But the natural history of a relapses is that it tends to evolve a little bit more slowly hours to days, and then resolves over a period of time. This relapsing remitting designation refers to these intermittent relapses within some potentially remission in the middle. I think the part that's deceiving about this name is that not everyone's rid of remits or comes completely back to normal though some people do.
The more relapses that an individual has, probably the more injury that's accumulated, the more neurologic symptoms that build up over time. When we take MRIs of individuals when they're having these relapses are attached, we usually see evidence of an active inflammatory MS plaque, usually somewhere in either the optic nerve, brain or spinal cord. Sometime can be multifocal, sometimes can just focal. That's relapsing-remitting multiple sclerosis. The majority of individuals that are diagnosed with MS usually are diagnosed with MS first. Then another sub-type of multiple sclerosis is called primary progressive multiple sclerosis.
If you look at the actual pathology on the brain across all these different types of MS, they will generally all look fairly similar. The difference in these designations is purely how someone presents clinically. Instead of these classics, you have a relapse and get better, have a relapse, get better. With primary progressive MS, people usually have slowly worsening symptoms from the onset. There can be active relapses that happen on top of the progression and we also call that active progressive multiple sclerosis.
But the main difference is that, and even in-between events, it seems like someone's clinically is still slowly worsening. Meaning instead of suddenly developing leg weakness they get better over a couple of weeks, it's a slow onset, you develop a little bit of foot drop and then more trouble walking and you're tripping and it slowly gets worse over time. Sometimes this can still stabilize out and plateau at some point in the future. But again, it's very unpredictable what this course may look like some time.
The other thing that characterizes it is that even if someone is getting clinically worse, we may not always see a clear correlate on imaging and this probably has to do with the fact that there are other types of nerve degeneration that's happening that we can't visualize as well with the technology we have. But likely there is radiographic progression that we can see along the way as well. The dreaded long-term course that can happen sometimes where individuals start out as relapsing-remitting MS over time can still potentially develop what we call secondary progressive MS, and so they have a clinical course that is relapsing-remitting MS for many years.
Then over time may then start to develop slow progression even in the absence of relapses, even in the absence of new active lesions that we can see on the MRI, and there's a portion of individuals who start out as relapsing-remitting MS, who may transition into secondary progressive MS. The goal of all of the therapies we have at this time is trying to slow down this process of progression or trying to prevent new lesion formation, trying to prevent relapses. But otherwise, this is the natural history of the subtypes of multiple sclerosis. The way that we make a diagnosis of multiple sclerosis, there have been new criteria. Basically, they revise this every few years, and our most recent diagnostic criteria are from 2017.
We call them the McDonald criteria. What we basically aim to meet is this criteria called dissemination in time and space. When you're trying to make a diagnosis of multiple sclerosis, you're trying to show evidence that there is an inflammatory disease process, that's one, happening across different parts of the central nervous system. Meaning we see lesions both in characteristic parts of the brain plus potentially characteristic parts of the spinal cord. Then you also want to see lesions that are at developing over time. Whenever I'm thinking about a patient and trying to make a diagnosis for the first time, I'm always thinking, okay, does this meet criteria for a dissemination in time and space? Most classically, if I see a 32-year-old that comes into the clinic and they have had an optic neuritis and then when I look back in their history or look at their MRIs, I also see what looks like likely an old spinal cord lesion and they have clinical symptoms suggestive of an old spinal cord lesion that may have recovered, right there you have what sounds like two events that affect two different parts of the central nervous system, and if you rule out all other causes right there, that individual would meet criteria for multiple sclerosis.
We've revised the criteria now just to allow people to be diagnosed earlier. They don't always have to have two clinical events. Sometimes if they've only had one clinical event and this is something that's characteristic of MS. If we take a look at their MRIs and we see that there's old lesions, even if they don't have clear evidence that they were asymptomatic from those lesions. We actually see this, not infrequently, where there's this silent progression where lesions can develop and because the injury is usually partial or there's a repair that the brain and spinal cord does on its own, someone may not have notable symptoms from it, or the symptoms were so mild that maybe they just ignored it in the past.
Even if they've had one clinical event but we see that there's evidence of old lesions elsewhere. Even if they didn't cause symptoms, that is sometimes enough to allow us to make a diagnosis as well. The goal of that is that we want to try to make a diagnosis of MS as early as we can because it does seem like intervening earlier in the process before we've given time for more injury to accrue and develop in the nervous system, likely the better chance we have of giving someone longer years of disability freedom or living a more normal life and function. The MRI or magnetic resonance imaging is our mainstay of diagnosing multiple sclerosis at this point, and so these are some characteristic findings that you might see on an MRI.
I'll just use some of these technical terms that we've learned. When we look at an MRI, when we're looking at these cross-sections, we call this an axial view, and when we're looking sideways, we call this a sagittal view. On this sequence of MRI, it's actually interesting because the gray matter actually looks white and the white matter looks gray, and so generally when you're looking at a brain MRI, usually the brain looks very symmetric. When there is asymmetries in the brain, they pop out right away.
You guys can probably see that here there's an area of white, we call it a hyperintensity that is not on the other side. Interestingly, when we look at MRIs, they're also flipped. This side is actually the right side and this is the left. This type of MS lesion, we call it a juxtacortical lesion. Very characteristic for MS where basically you have a white matter lesion that's just right about the cortex, so juxtacortical. Then we also have here periventricular lesions, meaning a lesion that's right up next to the ventricle of the brain, which is a normal open space in the brain.
Another area, very classic, we also call it Dawson's finger because it almost looks like fingers coming out from the ventricles of the brain. This is an example here of a classic spinal cord lesion. They tend to be ovoid.
They tend to be oriented towards the periphery of the spinal cord. Usually, doesn't involve the entire spinal cord, it's usually just partial, and so that's why sometimes people just have symptoms on one side of the body and not always both. Then they tend to be what we call short segment. Over here are the vertebral bodies of the spine and usually, MS lesions only span the length of one vertebral body. Not always, but generally speaking. Here are some other examples of classic MS lesions.
Again, another example of juxtacortical. Here's another view of those periventricular lesions that we saw, again you get that sense that it's like fingers projecting from the ventricles. We also see them in the lower part of the brain, which we call the brainstem. Then other examples here of a spinal cord lesion.
The other thing that we do when we get MRIs, if any of you guys have had an MRI before, you might recall that they put an IV in and they actually put contrast into your veins. Areas where there is active inflammation, you have a breakdown of the normal protective blood-brain barrier and so the contrast can seep through from the blood vessels into the brain. We will have contrast that lights up and that demonstrates to us that this is a lesion and active area of inflammation, likely within the last couple of weeks to even a couple of months, and that's suggesting something that's new, whereas something that's not enhancing is probably old. Hence you get that separation in time there.
Other diagnostic tools that we use is a lumbar puncture or also known as a spinal tap, where we basically take a small sample of fluid out of the lower part of the spine. We go at a level where your spinal cord is actually ended and it's just the nerve roots, so there's no risk of puncturing your spinal cord from doing a spinal tap. The goal of getting the spinal fluid is to look for evidence of inflammation and you can also rule out infection and other possible causes of this abnormal inflammation or neurologic symptoms. We also look for something called intrathecal gammaglobulin synthesis, which basically just means we're looking for abnormal antibody production within the spinal fluid. Generally, if we see abnormal antibody production that's specific and unique gesture the spinal fluid, meaning it's something happening in the central nervous system that's not happening in the rest of your immune system. That's something not necessarily specific to MS but is seen in probably 85-90 percent of people with MS.
It can also just be a general sign of inflammation, so we can see it in other neural immunologic conditions as well. But most classically, we call these oligoclonal bands. You may see that term come up.
If you guys have any history of working with anyone with MS or any background in neurology, you might have heard that term before. Something else that we use are what we call visual and somatosensory evoked potentials. We literally will put electric leads on people's heads and we will actually measure electrical activity from the stimuli, so how long does it take information to get from the eyes or from the hands and all the way to a certain part of the brain. You can actually just take time points and it gives you basically a speed of conduction at the end. Basically, if we see a slowing of how long it takes for information to get from one part of the body to the brain, that's usually a sign that there's probably some type of dim myelinating injury somewhere. If you imagine.
I'm measuring electrical conduction across a wire. If you were to take the insulation off a wire, the electrical conduction would probably be slow because it's not as efficient. It's the same idea, when there's demyelinating injury to the brain or spinal cord or to the optic nerve. Sometimes we also do this to help us confirm whether or not there's evidence of demyelination. It's a nice neurophysiologic correlate to what we see on imaging and so can also be a tool for helping us make a diagnosis of MS. Just a little bit
about the natural history of MS. As I said, the majority of people usually have an initial clinical attack or relapse followed by recovery, AKA having relapsing-remitting multiple sclerosis. The worry, of course, is even if you look well initially and recover initially from MS, there's an estimate that 58 percent of individuals that start out with relapse in MS do go on to develop secondary progressive MS and so this is in the absence of treatment. This is all data that gathered from before we had as potent of their abuse as we have now, and then it was estimated 66 percent of individuals would go on to develop secondary progressive MS 16-25 years down the line. That's always the worry again, why we always want to try to get individuals started on therapy is to hopefully delay this further or prevent secondary progressive MS from developing. As I said, there can be a long period of time between relapses sometime.
This one natural history study estimated maybe even up to almost two years between episodes. The main thing that they saw is that if someone developed vision loss or optic neuritis initially, they tend to have a longer period until their second episode. Slower progression seems to be related to having optic neuritis first. If you have a good recovery after your initial clinical event, that's probably better for you prognostically. The opposite of that would be if someone has a really bad initial relapse and doesn't recover, be worried that they might have a more aggressive course or their relapses may be more severe that they may not recover as well from them.
The longer time you have before your second relapse is probably a good prognostic indicator as well and then having fewer relapses in the first five years. You could imagine if someone was having a relapse every couple of months, that would be different from someone who's having a relapse every few years. Generally speaking, someone who's having relapses more frequently with multiple sclerosis, generally tend to be at higher risk for disability down the line. One thing I'll differentiate is how we treat a relapse from how we manage multiple sclerosis as a whole. When someone has a relapse, so they're having a new inflammatory event, they suddenly develop symptoms over the course of hours to days, they go to the emergency room. What generally happens is we treat them with high dose glucocorticoids.
Basically, steroids. Methylprednisolone is the intravenous medication that's used most often if someone goes to the emergency room. How we use practice and how we decide on treatment is all based on clinical trials. We try to use data-driven, scientific method driven recommendations as much as we can.
They actually did do a clinical trial on this. It was called the Optic Neuritis Treatment Trial, where they took individuals where they gave them a lower dose steroid by mouth versus a higher dose steroid into an IV and they found that the people who were given the IV steroids tended to get better faster. The one thing that they emphasize though, is that regardless of whether or not you gave them steroids, people usually recovered on their own to the same level regardless, but the steroids definitely helps them get better or recover from the relapse sooner. You can imagine if you can't see or you can't walk or your bladder function is off, you probably don't want to wait and see if it gets better over the course of months, you probably want to get better right away just because that really affects overall quality of life, so a point about that. Then one thing that we found recently is that if you dose steroids by mouth, that's the same dose, likely it seems like it's about as effective as it is if you give it by IV. Generally speaking, nowadays, especially if I'm treating someone in my clinic and I want to keep them out of the hospital and I think they're having a relapse, I might just treat them with high-dose steroids by mouth.
Then the other part of that is once we're done treating someone in the hospital setting for a new relapse with a new diagnosis, then we usually, after we've made a diagnosis of multiple sclerosis, want to put them onto a long-term maintenance therapy. Right now we don't have a cure for multiple sclerosis. Generally, we place patients on some type of maintenance therapy and the idea of that is just suppress the immune system to some degree to try to decrease this abnormal inflammation that drives new MS relapses and that drives progression and worsening of MS. They all have different mechanisms of action, which I'll go through very broadly here in the next slide. This is the timeline of multiple sclerosis therapies that have been FDA approved.
As Dr. Wong mentioned, we're really lucky in our field because over the last 30 years, basically every few years there's been a new MS therapy that's been developed and they all vary differently in their mechanisms of actions and how well they work. There's some of them that have similar mechanisms of action but maybe better tolerated from the side effect profile. I would say honestly, almost all of these therapies that I listed on here, even the ones that are all the way back from the early and mid '90s, I sometimes still use them. They're different enough that there could potentially be a place for almost any of these therapies.
There has been some therapies that have since been removed from our algorithm of use just because they ended up having severe side effects, etc., were found to be too dangerous. But generally speaking, any of the therapies that are on this list here, I still use in my practice in one form or the other. If we tear them, that's usually how I think about these disease-modifying therapies or immunotherapies and they have increasing efficacy as we go down these categories. The older medications, generally speaking, they're all injectable medications. Glatiramer acetate and interferons are just some of the names for these.
They're all medications that people would give themselves anywhere from like every once a week to a couple of times per week to every day and then after that they came out with different oral medications. Again, these oral medications also have different efficacies as well. Then the highest efficacy medications we have generally are what we call monoclonal antibodies and infusion-based medications. These monoclonal antibodies target specific parts of the immune system in different ways.
Some of them again, are more immunosuppressive than others. One way when I'm talking to my patients about what different therapies there are out there, I sometimes tear them in this way just to give them a framework of what therapies we may consider. Then as I mentioned, there's also many different mechanisms I will just give a couple of examples because I don't want to get too bogged down into this. But some examples would be some of the medications that we call the S1P receptor modulators.
They act on the receptors that prevent white blood cells from basically moving out of lymph nodes. If we actually measure someone's white blood cell count, what we call their lymphocyte count in their blood, it would actually look low and it's not because they are gone or we've destroyed them with the medication. It's actually just because those white blood cells are sequestered inside the lymph nodes and can't come out. We think that by preventing those white blood cells from getting out of the lymph nodes, it at least prevents the abnormal, the bad acting white blood cells from getting into the brain and spinal cord. Then another example would be a medication that we use very frequently here called Ocrelizumab. You will notice all the monoclonal antibodies end with the letters M-A-B MAB.
It targets something that we call CD20, which is found on the surface of a certain type of white blood cell called B-cells. By targeting that it leads to cell death of the B-cells depletes immature naive B cells suppresses the immune system to some degree and again, disrupts this potential inflammatory pathway. Other examples include medications that prevent white blood cells from proliferating. Some of them again lead to white blood cell depletion or death.
Some of them also Natalizumab is an example where it doesn't suppress the immune system but basically just targets something called Alpha4 integrin, which is found on white blood cells and which allows it to move across the blood-brain barrier from the peripheral immune system into the central nervous system. It doesn't actually deplete your immune system, but just prevents white blood cells from getting across the blood-brain barrier. Again, I won't get too much into that.
Usually, when I'm telling patients about these types of therapies, I'll probably choose a few options for them based on what their lifestyle goals are, how active their diseases, etc we'll go through a few of them. But just to give the sense that there's a really wide range of mechanisms of actions for these therapies. As I mentioned, it's a shared decision-making process between the physician and the patient for how we decide which therapy to go on. I often will spend usually like a 60-minute visit, sitting down with a patient after we've made the diagnosis, etc talked about this. It's certainly not an easy one.
I think when people imagine certain types of conditions, sometimes there's better algorithms for it where we say, if you have this condition, then we treat with x. It's a little bit more complex in multiple sclerosis because there's a lot of unknowns. We actually don't have a lot of scientific data right now to help us guide whether or not we need to put everyone on a really strong therapy early or not. For certain individuals, things that you might take into account is their age. Certainly, someone who's older, you might be more worried about putting on a lot of immunosuppression because they'll be at increased risk for infections compared to someone that's young. Similarly, if they have a lot of other medical conditions, they might be again, more at risk for infectious complications.
The type of multiple sclerosis they have plays into it. For instance, primary progressive MS really only has one approved therapy on there, whereas pretty much all of the therapies are approved for relapsing MS, but only one is formally approved for progressive MS so that makes a difference. The level of disease activity and other characteristics of the disease. If you get someone at initial presentation and you look at their MRI and they actually have a high burden of MS lesions and you suspect that they've probably had MS for a long time, you would probably be more inclined to put them on something that's high efficacy rather than one of those lower efficacy therapies.
Pregnancy planning comes a lot into trying to decide what treatments to put people on. Because almost all of these medications, it's difficult to be formally on when you're pregnant. We have to decide how do we dose a medication around your pregnancy? Because some medications are dosed just every six months. Some medications are dosed every day so we have to plan around that as well. There are certain medications that if we were to suddenly stop it when someone's pregnant can actually lead to worsening of MS disease activity. These are like the complex decision-making that goes into deciding what treatment to put people on and is oftentimes can be challenging to talk people through.
Even my colleagues who may be specialized in non-MS or non-neural immunologic conditions. I usually tell them if you don't feel comfortable advising on starting a therapy on your own, you're always welcome to refer to us for us to go through the pros and cons of each of them because it can be challenging. As I'd mentioned, we're trying to understand better, what types of treatment should we put people on early in the course of disease? At least right now, we tend to be moving towards starting people on higher efficacy therapies earlier. Potentially starting a high efficacy oral medication or one of these monoclonal antibodies early on in the treatment course.
There has at least been these retrospective observational studies that suggest that there's lower rates of disability progression when we do that. Very classically, even just like 5/10 years ago, it was I'm very typical that if someone was diagnosed with MS for the first time, you would right away start them on one of those lower-tier like first-tier MS options like the injectable medications, and then only escalate them further and further up if they had more disease activity or breakthrough disease or relapses. But the concern is that in doing that, you allow someone to develop more injury to the central nervous system over time. Now we tend to be a little bit more aggressive. Again, it's hard to make it a blanket statement because things differ so much individual by individual, but we overall tend to be leaning towards starting higher efficacy therapy earlier.
Then, just to say that there are certainly always new research that's going on in another mechanism of medication that's being looked at is something called the Bruton Tyrosine Kinase Inhibitors. Bruton tyrosine kinase or BTK is a critical molecule in intracellular signaling from B-cells and other types of white blood cells. When they've looked at this mechanism of medication in mouse models. There actually is a mouse model of multiple sclerosis is called EAE, where they have the mouse version of MS that's induced in them in the lab.
When they use these medications in the mouse models, it looks like it does lead to reduction in inflammation overall. The nice thing about these medications, as I had mentioned, there's all different forms, injectable forms, medication you take by mouth, medications that are given intravenously. The nice thing is that this would be a high efficacy, potentially a moderate to high efficacy therapy that would be dosed by the mouth. It's cheaper and probably easier to get to patients.
The other thing that's a little bit different from the other treatment options we have currently is that it seems to do a better job compared to the medications we have currently of getting across the blood-brain barrier. One of our concerns is that none of our MS medications are perfect. I would say they all have very modest efficacy against treating people that have progressive MS. By the time you're treating a patient,
if they already have progressive MS, it's harder to pull the brakes at that point. The hope is that this type of medication may do a better job of targeting and treating progressive MS. At this time we basically treat individuals with multiple sclerosis indefinitely. There are definitely certain treatment options that are thought of as more definitive treatment. Where the idea is you give someone a treatment for only a short period of time and they may not need treatment later down the line.
But again, even these treatment options haven't been shown to definitively halt multiple sclerosis. At this point, generally people are on some treatment for usually years and years. The decision to come off therapy is often based on maybe a patient just has never tolerated treatment. They've always had some type of side effect or maybe a very severe infection. It could be someone has had a very mild disease course and they've been very stable for 30 years. That might be a time to think about pulling medication off.
But again, we don't really have a lot of good research because we've only had MS treatments out for the last 30 years to really tell us when is the right time to stop. There are actually ongoing clinical trials that are looking at this. Hopefully, we'll have better data to guide our patients. Because, you know, at this point I have patients who are in their '60s, '70s, even '80s. It's oftentimes a very difficult decision to try to decide. Do we start therapy at this point? Do we potentially stop? Etc. The other thing we do day to
day in the clinic is also just monitoring individuals and taking care of them long-term. There are different parameters we use to help us decide whether or not someone is clinically stable or as stable as we can tell. One would be number of relapses. The goal would of course, be to have as minimal relapses as we can or maybe we get to the point where they're having no relapses at all. We use the MRI and our general surveillance as well and take a look at things like, are they developing new lesions? Do they have new active lesions? Something that we're trying to understand better is looking at overall brain atrophy and how we could use that to predict how someone is doing.
Then also the other thing is just having patients come to the clinic and examining them, looking to see how their strength did, how does it compare to last time? We have specific types of tests that we do that are also objective measures. One is the timed 25-foot walk. We actually just have someone walk 25 feet every time they come to the clinic and then we have a number that we can follow objectively like, "I'm starting to see that your walk is slowing down by 1 second, 2 seconds, etc."
That could be an early sign that someone is actually developing more progressive symptoms. The nine hole Peg Test and looked at hand dexterity. We look at visual acuity.
We do something called the symbol digit modality test, which is a test of cognition. Again, none of these markers are perfect and we are definitely looking for more ways that we can get even more granular and how we track individual's disease progression. But these are some of the examples of things that we do in the clinic currently. When we are looking at someone's overall disability, we actually use an extended disability scale. It goes from 0-10, 10 is essentially that, zero is no symptoms at all.
This is another way just to communicate to another neurologist. Just like when they look at the note, they can tell right away someone's overall level of disability. Up to a level of four, usually it means the symptoms are a fairly mild and may not be interfering as much with someone's day to day function. By the time you get to a four, usually that indicates someone has a limitation on how far they can walk.
When you get to an EDSS score of six, that's someone walking with a cane at that point. By 6.5, they're walking with a walker. You can imagine going above that you're getting 10 individuals who are then on wheelchair, even bed-bound, more severely disabled. Just to make a few points about once we make the diagnosis, what do people living with MS experience on a day to day.
It can be a myriad of neurologic symptoms and some of them more disabling than others, but just wanted to highlight some of them that may be a little bit more invisible. I think when you see someone walking with a walker, we see someone in a wheelchair that's a very visible sign to us that someone is disabled. But there's other ways of being disabled that may not be as visible. One is depression and anxiety. Depression and anxiety affects 20-50 percent of people living with multiple sclerosis. There's higher suicide rates in individuals with multiple sclerosis.
Suicidal ideations associated with age greater than 65, having bowel or bladder dysfunction, swallowing dysfunction, speech involvement. It's unclear whether or how much of the depression anxiety associated with something structural change that's happened in the brain versus a lot of the psychological factors that go along with having chronic illness. It's likely that it's a combination of both. But it's something that we always try to screen for when patients come to the clinic, it can really affect their overall quality of life.
Many patients perceive their overall disability level and function as worse if their depression or anxiety levels are high as well. Fatigue is another huge thing. This is definitely something that's seen in just chronic illnesses in general and especially chronic inflammatory illnesses.
It's reported and at least 75 percent of people living with multiple sclerosis leads to loss of work hours and leads to unemployment. I have patients even on disability for fatigue because it's just hard for them to tolerate getting through the day. Again, it's likely multifactorial from CNS damage, from medication side effects, from immunologic abnormalities, sleep changes, disability status and it's really hard to treat. But I find this extremely challenging for patients because they'll be very frustrated.
They'll feel like they're walking is fine and physically, if you examine them, they look overall well. But the problem is they just have severe fatigue and that can be extremely disabling, can contribute to depression, etc. The strategies for that is usually multifold.
One is telling them strategies for energy conservation. I always give the example of you're someone who can't charge your battery as full, like your phone battery just doesn't charge as much. You're someone who, you can't fill your gas tank up as much, so you just have to frequently recharge. You may be active, be productive for a few hours, but then need to rest and a lot of that listening to one's body and if they have the ability to rest when they can and I usually tell them to do that. Cognitive behavioral therapy is something else that people do to help them figure out strategies for energy contribution. Maximizing their sleep as much as we can.
I tell people to make lists. Say like you might have at one been able to do like 10 things on your to-do list for the day. Let's shorten that down to five. We also do use stimulants if all of these lifestyle modifications don't work.
All of them are off-label, but things that you might imagine that you use for ADHD, etc, we actually use some of those medications to treat MS related fatigue. This is just example of our treatment algorithm for looking at bladder dysfunction because that's another huge thing that we can see with multiple sclerosis and spinal cord injury in general is having difficulty with controlling the bladder. Potentially going too frequently or having urinary accidents or on the other side not being able to urinate or empty your bladder fully. I won't go through this necessarily because it's more medical, but just to show that we have different ways of managing this from a physical therapy standpoint and also medications you should take by mouth and also procedural standpoint. Then of course, the big thing is making sure that we're treating walking difficulties and falls. Walking is dependent on a lot of things.
It's both how much strength you have and endurance, but also balance, cognition, attention, all of that. People living with multiple sclerosis tend to have these types of issues with difficulty walking due to weakness and different things. The other thing that we tend to see very classically with MS is that people have a lot of foot drop. They just can't lift their foot at the ankle and as a result, they also tend to hyperextend their knees, so it can give a very characteristic type of walk. It can be challenging. You can imagine you would get very tired if one of your legs was bigger, you were dragging your foot.
It's also very easy to trip over your foot as well if you're not able to pick it up as much and that's a really huge reason for individuals to have falls. We recommend identifying early if someone has any issues with their walking or if their fall risk, getting them into physical therapy early, using some type of assistive device. I always tell patients, unfortunately, we can't always completely stop the progression of multiple sclerosis. But if we aren't able to, then we need to maximize safety.
We need to reduce risks for falls. If someone is falling alive, I'm going to tell them you need to be using a cane, you need to be using a walker, maybe even using a wheelchair more regularly. A lot of my patients often develop a lot of fatigue. As they walk further, they just noticed a lot of their neurologic symptoms come out further as they get fatigued and so that may be a reason to use something like a wheelchair or a motorized scooter. This is my last slide here. Just some other examples as well as MS related symptoms, spasticity.
Increased muscle tone, which is something we see from spinal cord injury, again in general, can be painful. It can also affect the ability to use a limb if it's very spastic, intense, and hard to move. Pain is very common. Anytime you've had injuries to the central nervous system, there's a dysregulation of how sensory input comes into the brain and that can be felt as very severe neuropathic pain.
Cognitive impairment. That's something we see in probably about 50 percent of individuals living with MS. It's usually not a classic like dementia type of memory loss though, there are some patients who have very severe in advance multiple sclerosis that can look very similar to more typical Alzheimer's dementia patient.
But most of the time it's more attention unrelated processing, thinking, processing related inefficiency and thinking, word finding difficulties. Then also sexual dysfunction, very common as well and can really affect individuals lives. With that I will wrap up here and I will end my SlideShare. I'm always struck by how the explosion of treatments for MS.
If again, back when I was in medical school really, we only had that first tier of medications that was low efficacy, so we were seeing a lot of disabled patients. Seems like most of the new therapies, even if they're not directly impacting the immune system, they're really affecting how the immune system is working. How has your treatment of MS patients changed during COVID, where we're worried about people who have lowered immune system? It's a really good question. I would say our initial strategy was to even think about taking people of therapy or delaying their retreatments, depending on how long they've had MS, how severe their disease is. Generally, people who are older, who are more stable, who had been on their medication for many years, those are individuals who are even like delaying treatment or even stopping, that pushed our hands sometime to say, hey, you're seven years old, you are in a high efficacy therapy.
You've actually been really stable for a long time. This might actually be a very reasonable period to pull back. There was certainly that decision that came in. There were individuals that I just tended to start on some of these not truly immunosuppressive therapies that were more immunomodulating. Like I talked about, there are certain options that don't really truly suppress the immune system and can still have pretty moderate to high efficacy.
If they were eligible for that, I did that. But then there was just the reality of there were individuals with really aggressive or active MS and we just didn't feel comfortable holding their therapy. It was just a risk-benefit ratio and we just told them to do as much as they could to minimize their risk from COVID-19 and probably still st
2022-04-17 21:17


